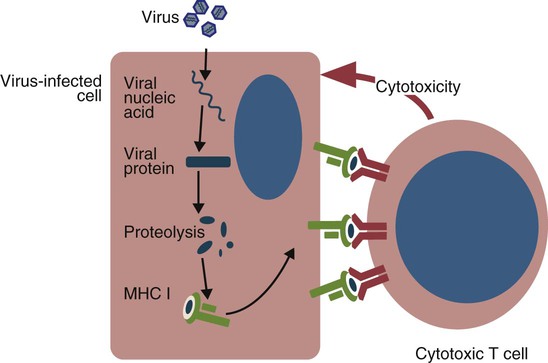
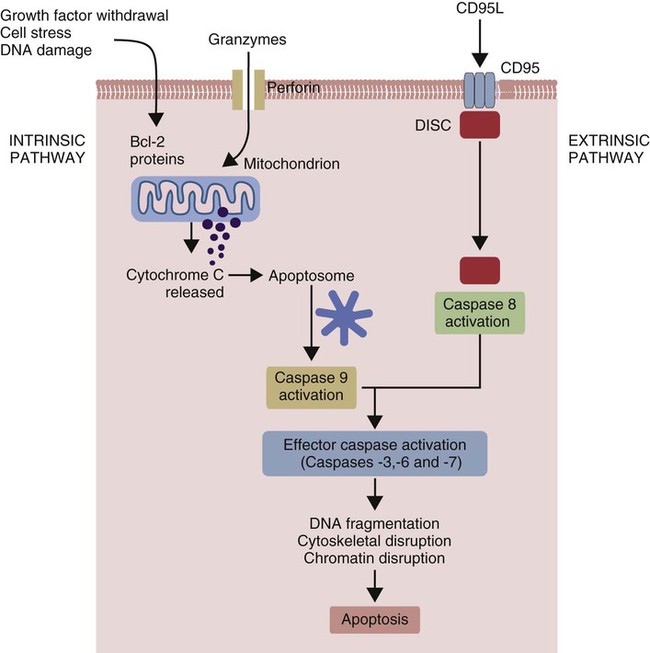
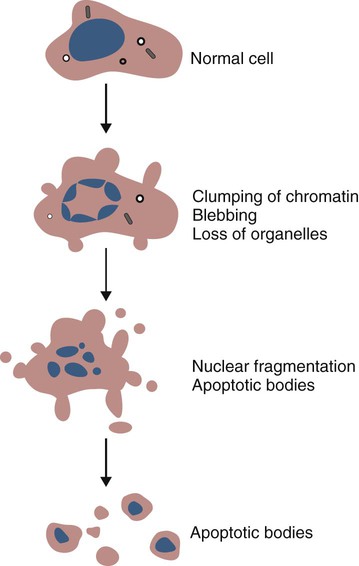
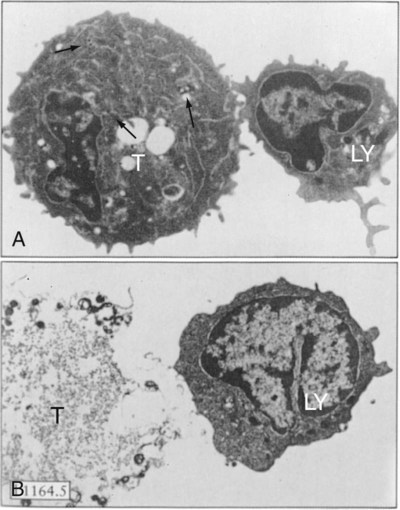
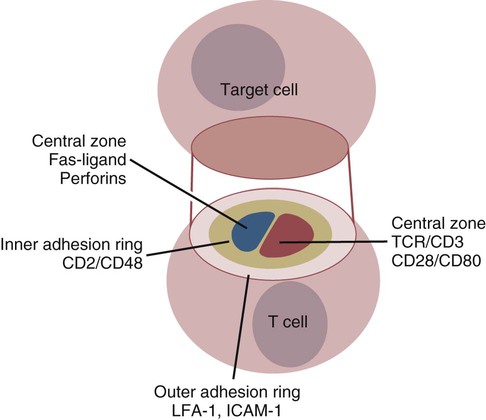
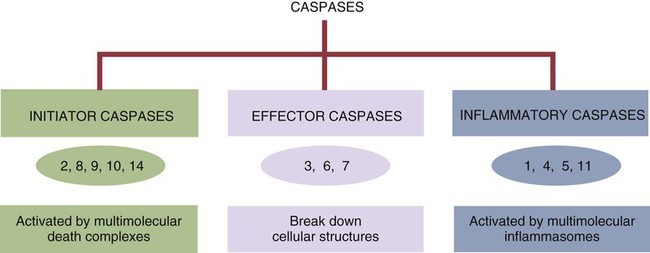
• Apoptosis is a mechanism whereby the body rids itself of unwanted cells. There are two major apoptotic pathways: one originates within the cell (intrinsic); the other is triggered by extracellular signals (extrinsic). Both result in the activation of an intracellular caspase cascade and eventual disassembly of cellular components. • Cell-mediated immune responses eliminate abnormal cells and intracellular organisms. • The elimination of abnormal cells involves the forced apoptosis of virus-infected target cells by cytotoxic T cells. • Cytotoxic T cells use two mechanisms to kill targets. They may trigger the extrinsic pathway using perforins and granzymes. Alternatively, they may trigger the intrinsic pathway using the death receptor Fas and its ligand. • Some bacteria and parasites may evade destruction by living within the endosomes of phagocytic cells, especially macrophages. • The elimination of these intracellular organisms is mediated by activation of M1 macrophages by interferon-γ (IFN-γ) produced by Th1 cells As described in Chapter 10, every time a cell makes a protein, a sample is processed and peptides are carried to the cell surface bound to major histocompatibility complex (MHC) class I molecules (Figure 18-1). If these peptides are not recognized by T cells, no response is triggered. If, however, the peptide-MHC complex binds T cell antigen receptors (TCR), then T cells will be triggered to respond. For example, when a virus infects a cell, T cells may recognize many of the peptides derived from the viral proteins. The T cells that respond to these endogenous antigens are CD8+. They use this CD8 to bind to MHC class I molecules on the target cells, thus promoting intercellular signaling and eventually killing the target cells. There are two major pathways of apoptosis. The extrinsic or death receptor pathway and the intrinsic or mitochondrial pathway. The death receptor pathway is triggered by cytokines such as tumor necrosis factor-α (TNF-α) acting through specific death receptors such as CD95 (Fas). Death receptors are a family of type 1 cell surface receptors that when activated trigger apoptosis. They all possess an 80-amino acid cytoplasmic sequence called a death domain. The most important of these death receptors are Fas (CD95) and the receptors for tumor necrosis factor (TNFR). Death receptors are activated by ligands commonly expressed on cytotoxic cells. The ligands bind to the death receptors and as a result assemble multiple adaptor proteins into a signaling complex. Once assembled, this complex activates initiator caspases-8 and -10 (Figure 18-2). The mitochondrial pathway, in contrast, is triggered by noxious stimuli that cause mitochondrial injury. The damaging stimuli (e.g., oxidants, radiation) activate proapoptotic bcl-2 proteins, which then cause the release of cytochrome C from mitochondria (Figure 18-3). The cytochrome C triggers the formation of a large multiprotein complex called an apoptosome. The apoptosome then activates initiator caspase-9. The initiator caspases activated by either pathway then trigger a cascade of “effector caspases” (caspase-3, -6, and-7) that degrade numerous proteins, activate endonucleases, break down organelles, and result in cell death and disassembly. The DNA of apoptotic cells characteristically breaks into many low-molecular-weight fragments. This fragmentation may be responsible for the characteristic way in which the nuclear chromatin condenses against the nuclear membrane (Figure 18-4). Affected cells shrink and detach from the surrounding cells. Eventually nuclear break-up and cytoplasmic budding form cell fragments called apoptotic bodies (Figure 18-5). Once fully activated, CD8+ T cells leave lymphoid organs and seek out infected cells by themselves. When they recognize an antigen expressed on another cell, the T cells will kill their target. Although most cells only undergo apoptosis after receiving very specific signals, cytotoxic T cells can induce apoptosis in any cell they recognize (Figure 18-6). When T cells encounter a target, an immunological synapse forms at the point of contact (Figure 18-7). This synapse has two “centers.” One part of the central zone contains the TCR-CD8 complex. The other serves as the portal of entry of T cell cytotoxic molecules into the target cell. Both are surrounded by a pSMAC rich in adhesion molecules that forms a “gasket,” preventing the accidental spill of cytotoxic molecules. Once a synapse forms, cytotoxic T cell killing is highly efficient. Within seconds after contacting a T cell, the organelles and the nucleus of the target show apoptotic changes, and the target is dead in less than 10 minutes. Cytotoxic T cells are also serial killers that can disengage and move on to kill other targets within 5 to 6 minutes.
T Cell Function and the Destruction of Cell-Associated Invaders
Endogenous Antigens
Apoptosis
Cytotoxic T Cell Responses
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree