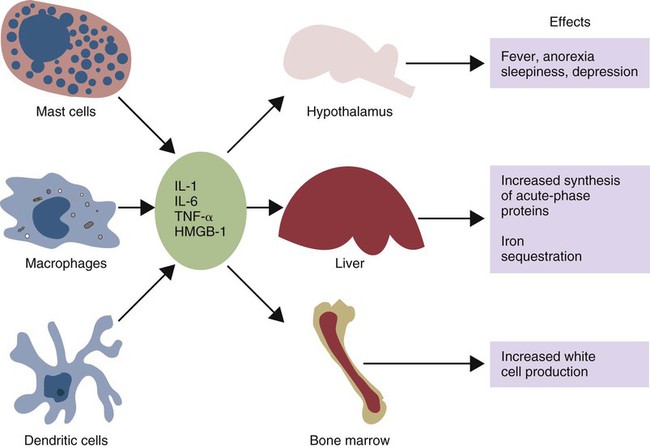
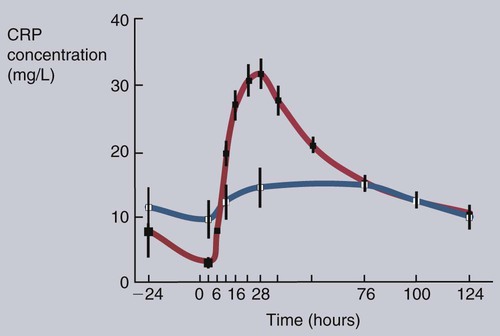
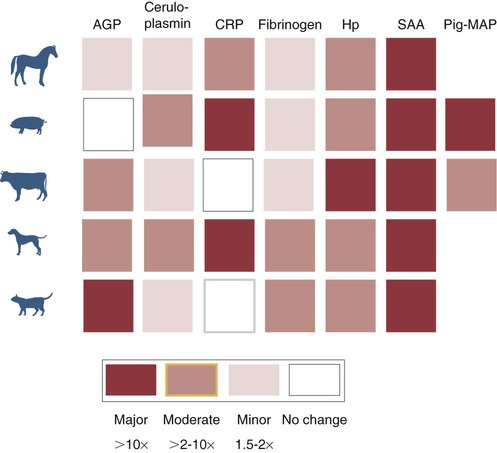
• Inflammation is not only a local tissue reaction but also a systemic reaction involving the whole body. • Cytokines secreted by sentinel cells cause a fever and are responsible for the diverse behavioral changes that we call sickness. • Excessive production of these cytokines (a cytokine storm) can lead to the development of a lethal shock syndrome. • Excessive, chronic release of inflammatory cytokines may result in tissue deposition of insoluble misfolded proteins called amyloid. When an animal is invaded by pathogens, a generalized response may occur—a response that we call sickness. The subjective feelings of sickness—malaise, lassitude, fatigue, loss of appetite, and muscle and joint pains—along with a fever, are signs of a systemic innate immune response. They reflect a change in the body’s priorities as it fights off the invaders. Microbial PAMPs acting through the pattern-recognition receptors (PRRs) of phagocytic cells stimulate the production of interleukin-1β (IL-1β), IL-6, and TNF-α. All three of these cytokines signal to the brain (Figure 6-1). They use two routes. One route is through the neurons that serve damaged tissue. IL-1 receptors are expressed on these sensory neurons, especially the vagus nerve. Sensory stimulation by IL-1β through the vagus nerve can trigger afferent signaling to the brain. (IL-1 will not trigger a fever if the vagus nerve is cut.) Lipopolysaccharide (LPS) and TLR4 can also trigger these vagal signals. They therefore trigger fever, nausea, and other sickness responses in the brain. The second route involves cytokines that either diffuse into the brain from the bloodstream or are produced within the brain. Microglial cells express TLR4, whereas neurons express TNF receptors. These cytokines can act on neurons or microglial cells to modify behavior and, for example, alter pain perception. As a result, anti-TNF antibodies can greatly reduce the pain associated with rheumatoid arthritis (Chapter 36). High-mobility group band protein-1 (HMGB1) (Chapter 3) is a potent sickness-inducing cytokine. Although IL-1, IL-6, and TNF-α have long been known to cause septic shock and sickness behavior, it is now clear that these three molecules induce HMGB1 release from macrophages several hours after initiation of sickness. It enters secretory lysosomes and is then released slowly from the cells. HMGB1 has been implicated in food aversion and weight loss by its actions on the hypothalamic-pituitary axis. It mediates endotoxin lethality, arthritis, and macrophage activation. The inflammation induced by necrotic cells is caused in part by the release of HMGB1 from disrupted nuclei and damaged mitochondria. Under the influence of IL-1β, TNF-α, and especially IL-6, liver hepatocytes increase protein synthesis and secretion. New proteins may also be synthesized in lymph nodes, tonsils, and spleen as well as in blood leukocytes. This increase begins about 90 minutes after injury or systemic inflammation and subsides within 48 hours (Figure 6-2). It may also occur following prolonged stress such as road transportation or confinement. Because this increase is associated with acute infections and inflammation, the newly produced proteins are called acute-phase proteins. About 30 acute-phase proteins have been recognized, and many are important components of the innate immune system. They include soluble PRRs, complement components, clotting molecules, protease inhibitors, and iron-binding proteins. Different mammals produce different sets of acute-phase proteins (Figure 6-3). Iron concentrations within animal tissues are normally very low. Mammalian blood has just 10−26 M free iron since almost all available iron is bound to proteins. These iron-binding proteins include transferrin, lactoferrin, hepcidin, siderocalin, haptoglobin, and ferritin. Because many pathogenic bacteria such as Salmonella species and mycobacteria require iron for growth, withholding iron through the use of potent iron-binding proteins is an effective innate defense mechanism (Box 6-1).
Systemic Responses to Inflammation
Sickness Behavior
Acute-Phase Proteins
Iron-Binding Molecules
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Systemic Responses to Inflammation
Only gold members can continue reading. Log In or Register to continue