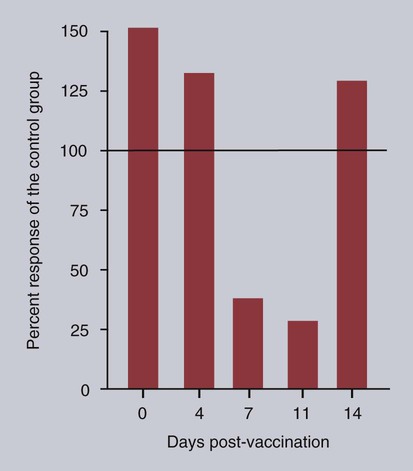
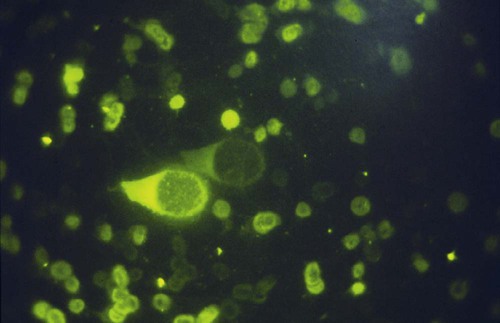
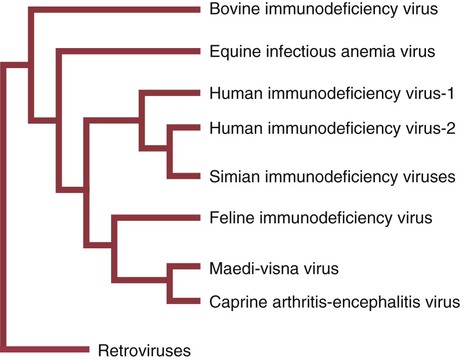
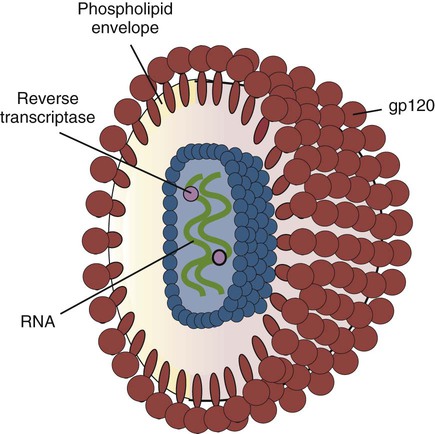
• Immunodeficiencies caused by damage to the immune system (secondary immunodeficiencies) are not uncommon in domestic animals. • The most important causes of immunosuppression are viral infections. To survive within a host, viruses may cause profound immunodeficiency either by infecting and killing lymphocytes or by causing them to become cancerous. • Other important causes of immunodeficiencies include stress, both physical and mental, some environmental toxins, malnutrition (both starvation and obesity), and old age. Loss of lymphocytes is common in virus infections since viral survival and persistence may require immunosuppression (Figure 38-1). Thus a lymphopenia occurs in feline panleukopenia, canine parvovirus-2 infection, feline leukemia, and African swine fever. Bovine virus diarrhea virus (BVDV) causes destruction of both B and T cells in the lymph nodes, spleen, thymus, and Peyer’s patches. As in canine distemper, surviving B cells fail to make immunoglobulins and respond poorly to mitogens. Viral destruction of Peyer’s patches causes intestinal ulceration and leads to secondary bacterial invasion. Both persistently infected cattle and normal cattle infected with cytopathic BVDV have depressed neutrophil functions, and bacterial clearance from the blood is impaired. A related virus, border disease virus, preferentially infects CD8+ T cells and interferes with their cytotoxic and immunoregulatory functions. The effect of some viruses on the immune system may be relatively complex or anomalous. In maedi-visna, a neurological disease of sheep caused by a retrovirus, cell-mediated immune responses such as graft rejection are suppressed, whereas B cell responses are enhanced (Chapter 26). Some leukemia viruses may be selectively immunosuppressive, so that depression of the IgG response is greater than that of the IgM response. In equine infectious anemia, the IgG3 response is variably depressed, whereas synthesis of the other immunoglobulin classes remains unaffected. Feline leukemia virus (FeLV) is an oncogenic retrovirus that can cause both proliferative and degenerative diseases in cats (Figure 38-2). Three naturally occurring viral variants are recognized based on the structure of their gp70 protein. FeLV-A is the predominant, naturally transmitted variant. It is present in all FeLV-infected cats. The other variants are only found in association with FeLV-A. When FeLV-A combines with endogenous retroviral sequences (sequences that are stably integrated into the cat genome and are normally never expressed but are genetically transmitted), FeLV-B is formed. FeLV-B is found in about 50% of viremic cats and has a greater propensity than FeLV-A to cause tumors. FeLV-C is found in about 1% to 2% of infected cats and arises from FeLV-A through a mutation in the envelope gene. It is much more suppressive for bone marrow than FeLV-A. The introduction of sensitive molecular diagnostic techniques to replace serologic assays has changed our views on persistent FeLV infections. Real-time and reverse-transcriptase polymerase chain reaction (PCR) assays are much more sensitive and specific than virus isolation, antigen detection, or immunofluorescence. These tests have shown that many cats may have FeLV DNA integrated into their cells but never develop an antigenemia. Vaccines may be able to prevent the development of clinical disease but not proviral integration. These latent infections may persist for years, and viremia or disease develops occasionally. On the other hand, this integrated viral DNA may also be required for long-term protection. Other cats may have both detectable viral nucleic acids and antigenemia (i.e., active infections). FeLV antigenemia may be detected by an antigen-capture ELISA, by the membrane filter technique, or by rapid immunochromatography of blood or serum. A direct immunofluorescent test on a buffy coat smear using antibodies to group-specific antigen can detect cell-associated antigen and hence intracellular viremia (Figure 38-3). Alternative testing methods include testing saliva or tears using material collected on a swab or filter paper strips. Feline immunodeficiency virus (FIV) was originally isolated from cats with clinical immunodeficiency. The virus is an enveloped, single-stranded RNA virus belonging to the lentivirus subgroup of retroviruses. It is differentiated from FeLV (a γ-retrovirus) by the biochemical requirements of its reverse transcriptase. (FIV reverse transcriptase requires magnesium, whereas FeLV requires manganese.) FIV is related to HIV, the cause of AIDS (Figure 38-4). FeLV and FIV are distinctly different viruses, and antibodies made against one do not react with the other. Nevertheless, approximately 12% to 33% of FIV-infected cats may also be infected with FeLV, an especially potent immunosuppressive mixture. At least five different genetic clades (or subtypes) of FIV have been identified. Subtype variations may account for differences in pathogenicity, tissue tropism, and clinical disease.
Secondary Immunological Defects
Virus-Induced Immunosuppression
Retrovirus Infections in Cats
Feline Leukemia
Diagnosis
Feline Immunodeficiency Virus
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Secondary Immunological Defects
Only gold members can continue reading. Log In or Register to continue