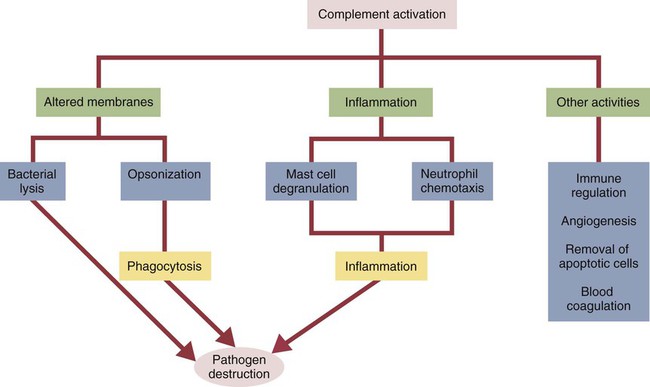
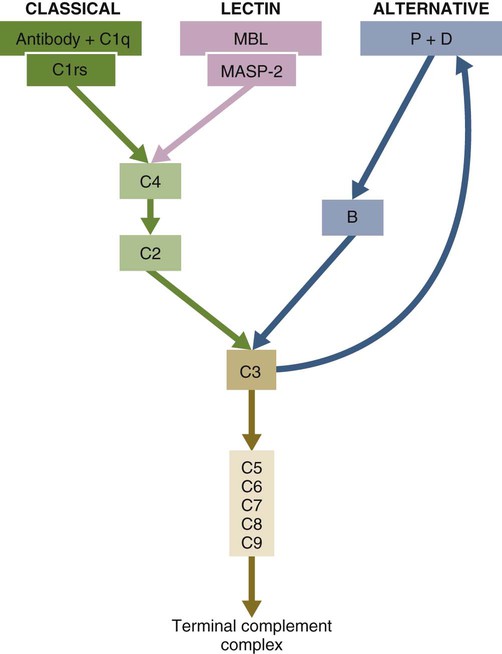
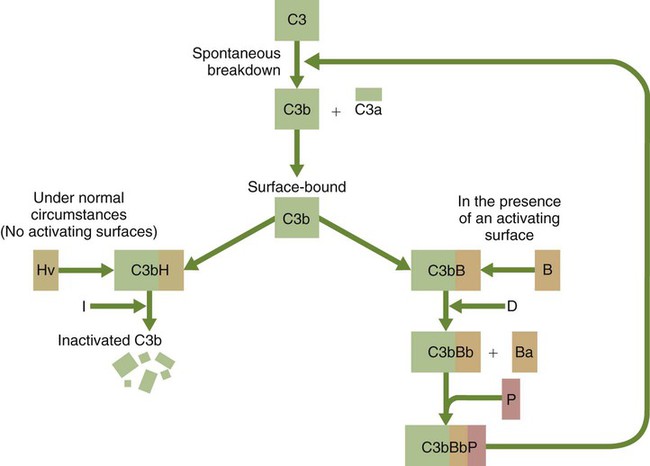
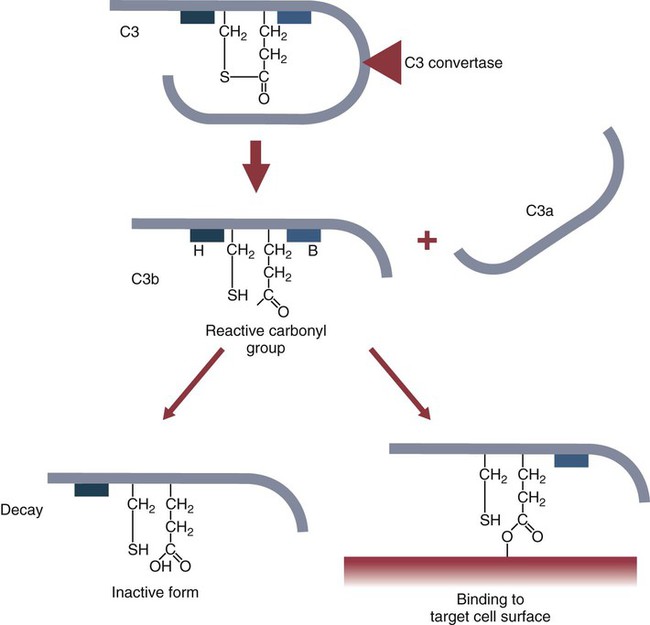
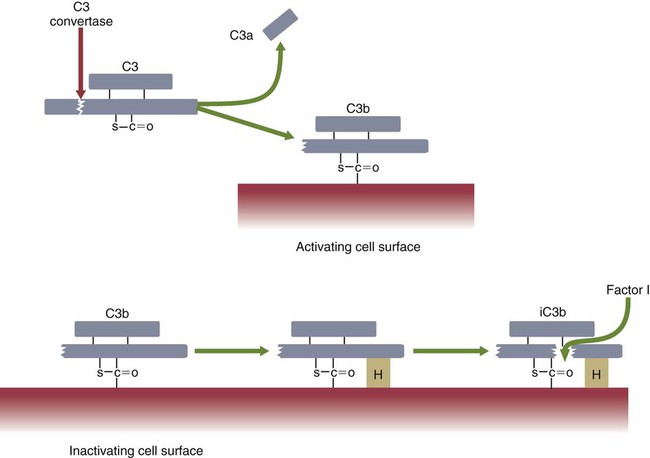
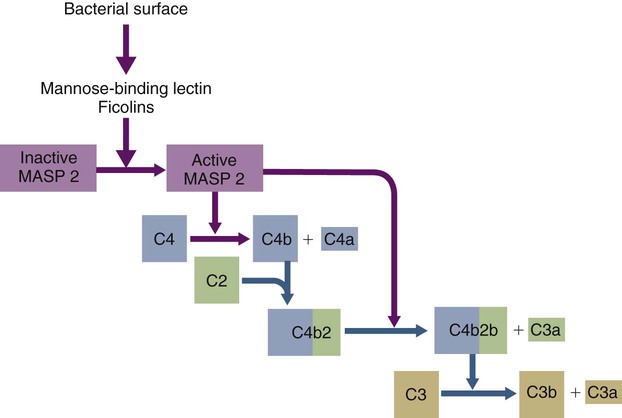
• The complement system plays multiple roles in both innate and adaptive immunity. • Complement proteins are found in normal serum. • The complement system may be activated through three different pathways: two innate pathways, the alternative pathway and the lectin pathway, and one adaptive pathway, the classical pathway. • The innate pathways are activated by recognizing microbial pathogen-associated molecular patterns (PAMPs). The classical pathway is activated by antibodies bound to foreign antigens. • Complement components, especially C3b, bind covalently to invading microbes and opsonize them. • Complement components may form a terminal complement complex and punch holes in microbes. • The complement system triggers inflammation through the release of the potent chemoattractant C5a. • Deficiencies of some complement components lead to increased susceptibility to infections. Protection from infection requires that the innate immune system respond to invasion as rapidly as possible. An important component of this early response is the complement system. The complement system is a complex interacting network that consists of many interacting pattern-recognition proteins, proteases, serum proteins, receptors, and regulators (Figure 7-1). Complement proteins are activated through pathways that cause some molecules to bind covalently (and hence irreversibly) to the surface of invading microbes. Once bound, these proteins can destroy the invaders. The complement system is inactive in healthy, uninfected animals. It is activated by either pathogen-associated molecular patterns (PAMPs) on the surface of infectious agents or by antigen-bound antibodies. Because the complement system is so potent, it must be carefully regulated and controlled. This in turn makes for significant complexity. The complement system consists of sets of inactive proteins that are activated in a stepwise manner. Three major steps are involved. First, the complement system must be activated. Second, a key protein called C3b must be generated. Third, a terminal complement complex is assembled through an amplification pathway. Once activated, the complement system generates multiple effector molecules. The progression of complement activation and the delivery of these effector molecules are carefully regulated. The first step, the triggering of complement activation, can occur by three different mechanisms, referred to as the alternative, the lectin, and the classical pathways (Figure 7-2). The alternative and lectin pathways are activated directly by microbial carbohydrates—typical examples of the pattern-recognition pathways that trigger innate immunity. The classical pathway, in contrast, is an evolutionary recent pathway activated when antibodies bind to the surface of an organism and thus works only in association with adaptive immune responses. Although complement has been conventionally regarded as a series of linear pathways, like other areas of immunology, it should properly be regarded as a network with several key hubs. The 30 or more proteins that form the complement system are either labeled numerically with the prefix C (e.g., C1, C2, C3) or designated by letters of the alphabet (B, D, P, and so forth). Some are found free in serum, whereas others are cell-surface receptors. Complement components account for about 5% to 10% of the proteins in blood serum. The size of complement components varies from 24 kDa for factor D to 460 kDa for C1q. Their serum concentrations in humans vary between 20 µg/mL of C2 and 1300 µg/mL of C3 (Table 7-1). Complement components are synthesized at multiple sites throughout the body. Most C3, C6, C8, and B components are made in the liver, whereas C2, C3, C4, C5, B, D, P, and I are made by macrophages. Neutrophil granules may store large quantities of C6 and C7. As a result, these components are readily available for defense at sites where macrophages and neutrophils accumulate. Table 7-1 In healthy normal animals, C3 breaks down slowly but spontaneously into two fragments called C3a and C3b (Figure 7-3). This opens up the C3b molecule to expose the thioester group. The thioester then generates a carbonyl group that binds the C3b irreversibly to carbohydrates and proteins on nearby cell surfaces (Figure 7-4). The breakdown of C3 also exposes binding sites for a protein called factor H. When factor H binds to these sites, a protease called factor I degrades the C3b, blocking further activity and generating two fragments, iC3b and C3c. iC3b binds receptors found on circulating leukocytes (Figure 7-5). It stimulates these cells to engulf pathogens and activate inflammatory cells. The final breakdown product of C3, C3dg, targets pathogens to surface receptors on B cells and so promotes antibody production (Chapter 15). The second method of activating the complement system involves the use of soluble pattern-recognition molecules that recognize microbial carbohydrates. These lectins bind to microbes and then activate proteases that activate complement. Like the alternative pathway, this is an innate pathway triggered simply by the presence of bacterial PAMPs (Figure 7-6).
Innate Immunity
The Complement System
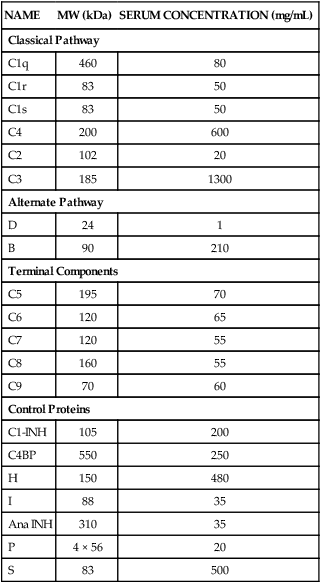
Complement Proteins
NAME
MW (kDa)
SERUM CONCENTRATION (mg/mL)
Classical Pathway
C1q
460
80
C1r
83
50
C1s
83
50
C4
200
600
C2
102
20
C3
185
1300
Alternate Pathway
D
24
1
B
90
210
Terminal Components
C5
195
70
C6
120
65
C7
120
55
C8
160
55
C9
70
60
Control Proteins
C1-INH
105
200
C4BP
550
250
H
150
480
I
88
35
Ana INH
310
35
P
4 × 56
20
S
83
500
Activation Pathways
The Alternative Pathway
The Lectin Pathway
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Innate Immunity: The Complement System
Only gold members can continue reading. Log In or Register to continue