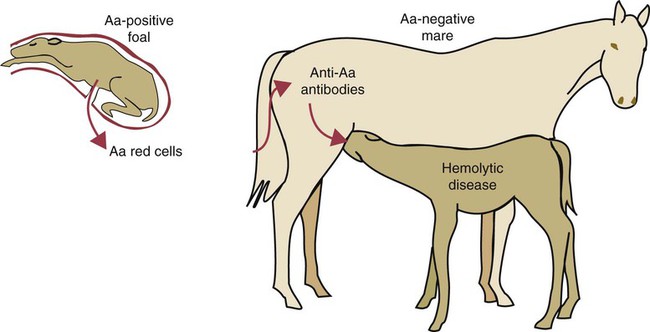
• Type II hypersensitivity, also called cytotoxic hypersensitivity, occurs when antibodies (and complement) destroy normal cells. • The destruction of transfused red blood cells when administered to a mismatched recipient is an example of type II hypersensitivity. The resulting disease is due to the lysis of the transfused red cells by antibodies and complement. • Mothers may become sensitized by their fetus during pregnancy and make antibodies against fetal red cells. These antibodies, if ingested in colostrum, may cause destruction of a newborn animal’s red cells. This is called hemolytic disease of the newborn. • Some vaccines may induce anti-MHC antibodies in cows. If ingested in mother’s colostrum, these antibodies may cause a lethal pancytopenia in their calves. • Some drugs may bind to blood cells and make them targets of antibodies in a type II hypersensitivity reaction. Animals may possess antibodies against foreign blood group antigens even though they may never have been exposed to foreign red cells. For example, J-negative cattle have anti-J antibodies in their serum, and A-negative pigs have anti-A antibodies. These “natural” antibodies (or isoantibodies) are derived not from previous contact with foreign red cells but from exposure to cross-reacting epitopes that are commonly encountered in nature (see Figure 9-8). Many blood group antigens are also common structural components of plants, the intestinal microbiota, protozoa, or helminths. The presence of these natural antibodies is not, however, a uniform phenomenon, and not all blood group antigens are accompanied by the production of natural antibodies to their alternative alleles. All mammals possess red cell antigens that can affect blood transfusions and on occasion cause HDN in newborn animals (Table 29-1). Although historically they were named alphabetically in order of their discovery, there is a growing tendency to add the prefix EA (erythrocyte antigen) to reduce confusion with MHC antigens. Table 29-1 Horses possess seven internationally recognized blood group systems (EAA, EAC, EAD, EAK, EAP, EAQ, and EAU.) Some, such as EAC, EAK, and EAU, are simple, one-factor, two-allele, two-phenotype systems. On the other hand, the EAD system is very complex, with at least 25 alleles identified to date. Their major significance lies in the fact that HDN in foals is relatively common (Figure 29-1). In mules, in which the antigenic differences between dam and sire are great, about 8% to 10% of foals may be affected. In thoroughbreds and standardbreds, the prevalence is considerably less, ranging from 0.05% to 2% of foals. This is despite of the fact that in up to 14% of pregnancies, the mare and the stallion have incompatible red cells.
Red Cell Antigens and Type II Hypersensitivity
Blood Groups
Blood Groups, Blood Transfusion, and Hemolytic Disease in Domestic Animals
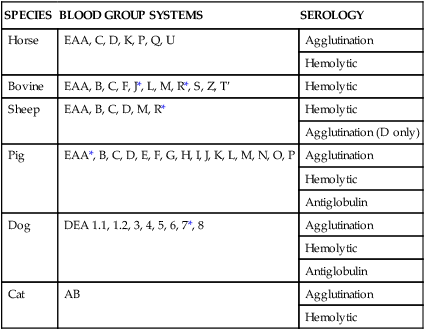
SPECIES
BLOOD GROUP SYSTEMS
SEROLOGY
Horse
EAA, C, D, K, P, Q, U
Agglutination
Hemolytic
Bovine
EAA, B, C, F, J*, L, M, R*, S, Z, T′
Hemolytic
Sheep
EAA, B, C, D, M, R*
Hemolytic
Agglutination (D only)
Pig
EAA*, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P
Agglutination
Hemolytic
Antiglobulin
Dog
DEA 1.1, 1.2, 3, 4, 5, 6, 7*, 8
Agglutination
Hemolytic
Antiglobulin
Cat
AB
Agglutination
Hemolytic
Horses
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Red Cell Antigens and Type II Hypersensitivity
Only gold members can continue reading. Log In or Register to continue