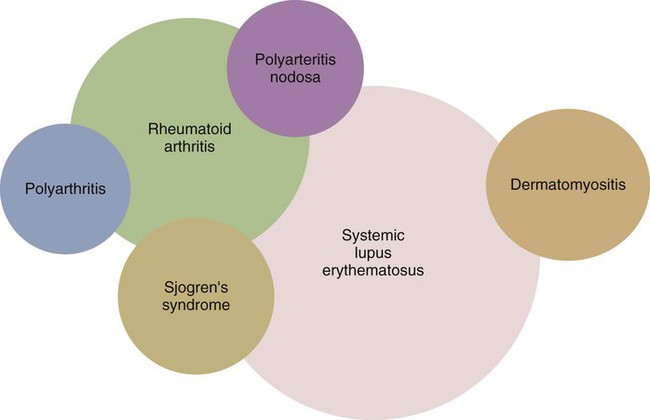
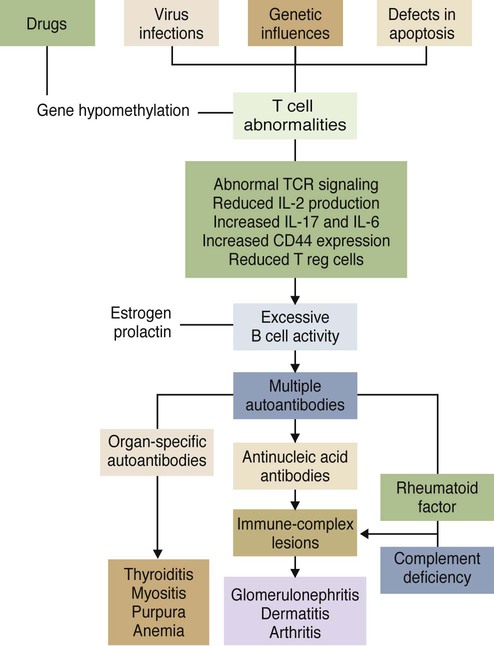
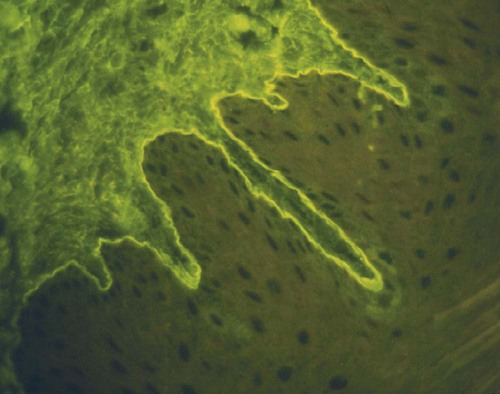
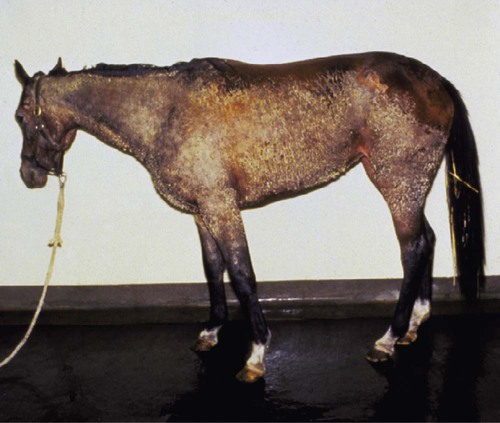
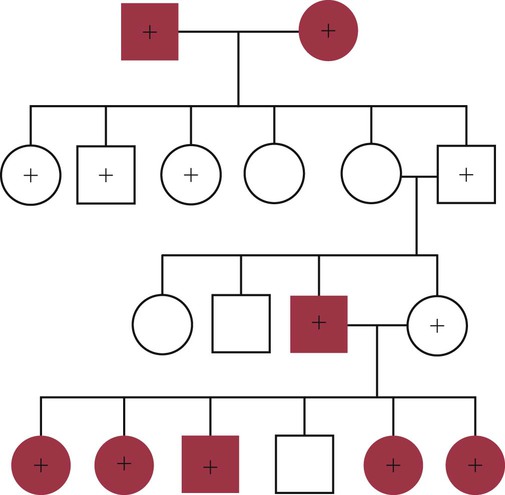
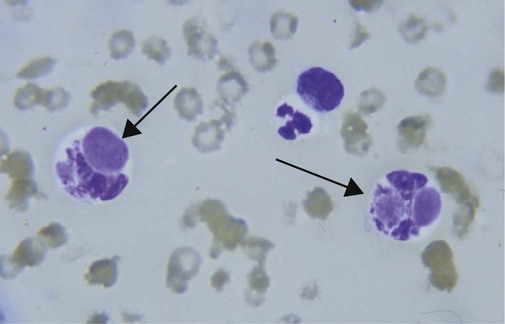
• Some autoimmune diseases may result from immunological attack on many different organs at the same time. This probably reflects a major loss of control of the innate and adaptive immune systems. • Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by multiple autoimmune responses together with the presence of autoantibodies to nuclear antigens. Lupus may represent several different disease entities. • Many different forms of arthritis may also be immunologically mediated. The most significant of these is the erosive arthritis called rheumatoid arthritis. It is associated with the development of autoantibodies to joint components, such as collagens, and of rheumatoid factor (RF), an autoantibody against immunoglobulin G (IgG). • There is a significant clinical overlap among many of these syndromes, making precise diagnosis difficult. Animals may suffer from complex inflammatory diseases that involve multiple organ systems. In human medicine, these have been called “rheumatic” diseases, “connective tissue” diseases, or “collagen” diseases based on outdated views on their pathogenesis. These systemic diseases or syndromes are interrelated and have many overlapping clinical features (Figure 36-1). One common feature is extensive and uncontrolled inflammatory responses, and it may be useful to consider them to be forms of innate autoimmunity or “autoinflammatory diseases.” Because of their many similarities, it is sometimes difficult to come to a definitive clinical diagnosis. SLE is a complex disease syndrome (or perhaps even multiple diseases) that has been described in humans, other primates, mice, horses, dogs, and cats. It is characterized by a broad and bewildering diversity of different symptoms and a wide variety of disease courses as symptoms flare and recede over time. The factors that lead to the development of lupus are complex, multifactorial, and poorly defined (Figure 36-2). Its development is affected by environmental factors, including infectious agents, drugs, and food, in association with the combined effects of many different genes. Patients develop a variety of autoantibodies, changes in T cell function, defective phagocytosis, impaired apoptosis, multiorgan inflammation, and oncogene expression. The hallmark of all forms of lupus is the development of autoantibodies against many different nuclear structures, including nucleic acids, ribonucleoproteins, and chromatin. These antinuclear antibodies (ANAs) are found in 97% to 100% of dogs with lupus compared with 16% to 20% of normal control animals. About 16 different nuclear antigens have been described in humans. Dogs differ from humans in that they mainly develop autoantibodies against nuclear proteins such as histones and ribonucleoproteins. These antinuclear antibodies cause tissue injury by several mechanisms. They can combine with free antigens to form immune complexes that are deposited in glomeruli, causing a membranoproliferative glomerulonephritis (Chapter 30). They may be deposited in arteriolar walls, where they cause fibrinoid necrosis and fibrosis, or in synovia, where they provoke arthritis. They may bind to Fc receptors on immune cells, leading to cell activation. The immune responses in SLE are associated with the production of interferon- α (IFN-α) so that the level of this cytokine correlates with disease activity. IFN-α is produced by plasmacytoid dendritic cells. It is believed that in SLE, FcR-, and TLR-mediated uptake of immune complexes and nucleic acids activates plasmacytoid dendritic cells. The IFN promotes inflammatory responses, activating macrophages and autoreactive T cells. ANAs also bind to the nuclei of degenerating cells to produce round or oval structures called hematoxylin bodies in the skin, kidney, lung, lymph nodes, spleen, and heart. Within the bone marrow, opsonized nuclei may be phagocytosed, giving rise to lupus erythematosus (LE) cells (Figure 36-3). A related defect in some lupus patients appears to be the impaired clearance of apoptotic cells. Normally, apoptotic cells are removed by phagocytosis by macrophages without causing inflammation (Chapter 5). Macrophages from SLE patients, however, show defective phagocytosis of apoptotic cells, which as a result accumulate in tissues. Nuclear fragments from these cells may be trapped and processed by dendritic cells, thereby triggering autoantibody formation. The defect is most obvious in the skin of affected animals, where ultraviolet (UV) radiation leads triggers apoptotic cell death. Nucleic acids from these cells may then act as autoantigens and trigger autoantibody formation. These autoantibodies in turn lead to immune complex deposition and tissue damage. Complement components mediate the efficient clearance of apoptotic cells, so complement deficiencies are associated with the development of lupus-like syndromes. Although a failure of apoptosis leading to activation of autoimmune B cells and multiple autoimmune disorders is a feature of lupus, its initiating cause remains obscure. Although ANAs are characteristic of lupus, many other autoantibodies are produced, suggesting that affected animals may have grossly abnormal B cell function. Affected animals show abnormalities in B cell signaling and migration, overexpression of CD154 (CD40L), and enhanced production of interleukin-6 (IL-6) and IL-10. Some experimental mouse models show overexpression of B cell stimulatory molecules by T cells and dendritic cells. It is therefore possible that the production of multiple autoantibodies in lupus is a combined result of defective apoptosis, overstimulation of B cells, and a failure to eliminate self-reactive B cells. Autoantibodies to red cells induce a hemolytic anemia. Antibodies to platelets induce a thrombocytopenia. Antilymphocyte antibodies may interfere with immune regulation. About 20% of dogs with lupus produce antibodies to IgG (RFs). Antimuscle antibodies may cause myositis, and antimyocardial antibodies may provoke myocarditis or endocarditis. Antibodies to skin basement membrane cause a dermatitis characterized by changes in the thickness of the epidermis, focal mononuclear cell infiltration, collagen degeneration, and immunoglobulin deposits at the dermoepidermal junction. These deposits form a “lupus band,” seen in many other autoimmune skin diseases in addition to lupus (Figure 36-4). In humans, lupus skin lesions are commonly restricted to the bridge of the nose and the area around the eyes since apoptosis is triggered by UV radiation in sunlight. The results of this excessive immune reactivity are also reflected in a polyclonal gammopathy, enlargement of lymph nodes and spleen, and thymic enlargement with germinal center formation. As described in Chapter 7, some lupus patients have a deficiency of the complement receptor CD35. As a result, immune complexes are not bound to red cells or platelets and therefore are not effectively removed from the circulation. These immune complexes may then be deposited in the glomeruli or in joints. Equine lupus presents as a generalized skin disease (alopecia, dermal ulceration, and crusting), accompanied by an antiglobulin-positive anemia. The disease is remarkable insofar as affected horses may be almost totally hairless (Figure 36-5). Affected horses are ANA positive, although LE cell tests are equivocal in this species. Skin biopsies show basement membrane degeneration and immunoglobulin deposition typical of lupus. Affected horses may also have glomerulonephritis, synovitis, and lymphadenopathy. Treatment of reported cases has been unsuccessful. Lupus affects middle-aged dogs (between 2 and 12 years of age) and affects males more than females. The disease is commonly seen in Collies, German Shepherds, Nova Scotia Duck Tolling Retrievers, and Shetland Sheepdogs, but Beagles, Irish Setters, Poodles, and Afghan Hounds are also affected. Lupus (or positive lupus serology) may occur in related animals, supporting the importance of genetic factors. For example, dogs possessing the MHC class I antigen DLA-A7 are at increased risk and those possessing DLA-A1 and -B5 are at decreased risk for developing disease (Figure 36-6). When dogs affected by lupus are bred, the number of affected offspring is higher than can be accounted for genetically, suggesting that the disease may be vertically transmitted. A type C retrovirus has been suggested as a potential trigger of lupus. A simple diagnostic rule for lupus could be stated as follows: Suspect lupus in an animal with multiple disorders such as those described previously and either a positive test for ANA or a positive test for LE cells (Box 36-1). ANAs are normally demonstrated by immunofluorescence. Cultured cells or frozen sections of mouse or rat liver on a microscope slide are used as a source of antigen. Dilutions of a patient’s serum are applied to this, and the slide is incubated and then washed off. Binding of ANA to the cell nuclei is revealed by incubating the tissue in a fluorescein-labeled antiserum to canine or feline immunoglobulins and then rewashing. Several different nuclear staining patterns have been described for humans and their clinical correlations identified. In animals, staining patterns have been less thoroughly investigated, and their significance is less clear. Evidence suggests that a homogeneous staining pattern or staining of the nuclear rim is of greatest diagnostic significance but that nucleolar fluorescence is not (Figure 36-7). Dogs whose serum generates a speckled fluorescence pattern tend to have autoimmune diseases other than lupus. Some normal dogs, dogs undergoing treatment with certain drugs (griseofulvin, penicillin, sulfonamides, tetracyclines, phenytoin, procainamide), and some dogs with liver disease or lymphosarcoma may have detectable ANAs. ANAs are also found in a significant proportion of dogs infected with Bartonella vinsonii subsp. berkhoffii, Ehrlichia canis, and Leishmania infantum. Dogs infected with multiple vector-borne organisms are especially likely to be ANA positive.
Systemic Immunological Diseases
Systemic Lupus Erythematosus
Pathogenesis
Equine Lupus
Canine Lupus
Diagnosis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Systemic Immunological Diseases
Only gold members can continue reading. Log In or Register to continue