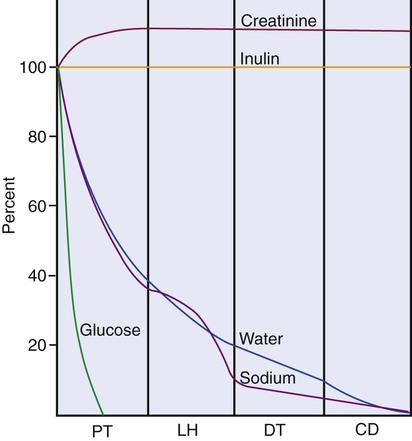
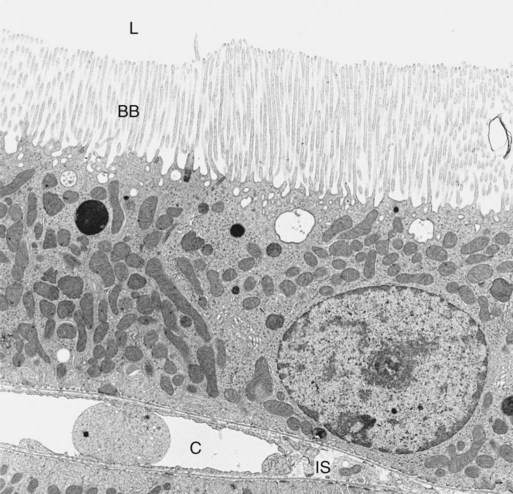
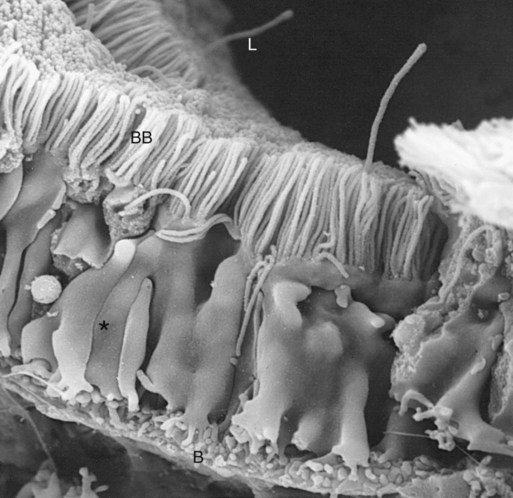
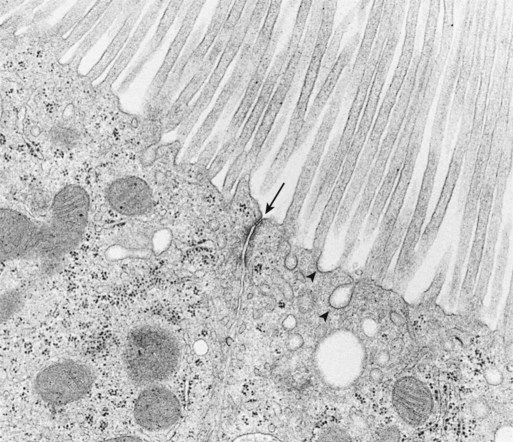
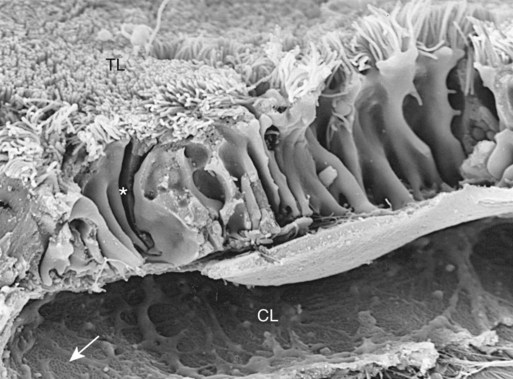
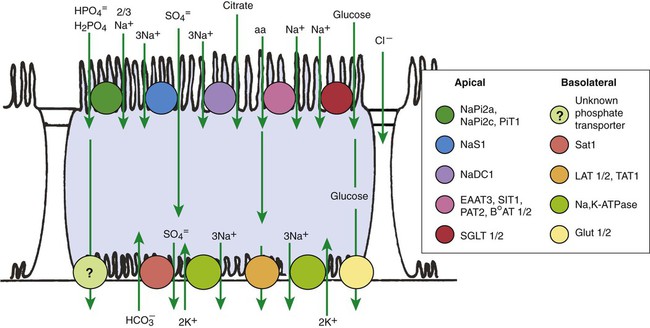
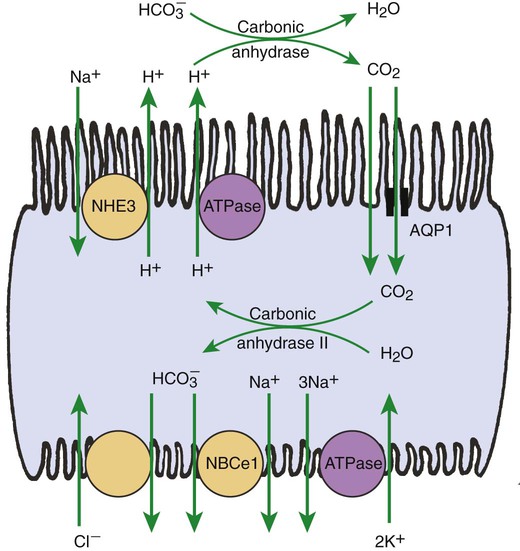
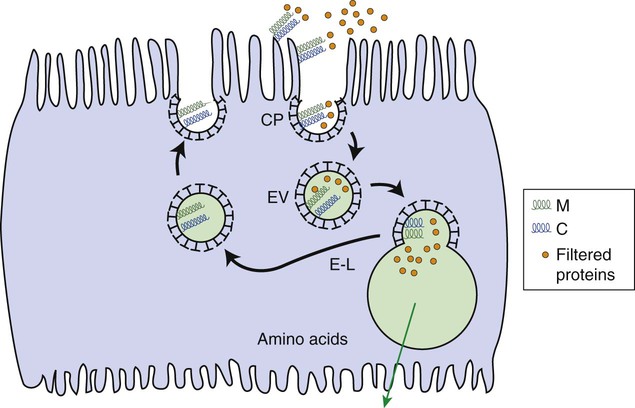
1. The renal tubule reabsorbs filtered substances. 2. Renal tubule function may be assessed by determining fractional excretion rate. 3. The proximal tubule reabsorbs the bulk of filtered solutes. 4. The proximal tubule secretes organic ions. 5. The thick ascending limb and distal convoluted tubule reabsorb salts and dilute the tubule fluid. 6. The collecting duct reabsorbs sodium chloride and can secrete or reabsorb potassium. 7. Solute transport is regulated by systemic and intrarenal signals. 8. Angiotensin II stimulates sodium uptake in proximal tubule, distal nephron, and collecting duct. 9. Aldosterone enhances sodium reabsorption and potassium secretion. 10. Other hormones and ligands that regulate sodium transport include antidiuretic hormone, nitric oxide, endothelin-1, and atrial natriuretic peptide. 11. Phosphate uptake in the proximal tubule is decreased by parathyroid hormone. 12. Calcium reabsorption in the distal nephron and connecting segment is enhanced by parathyroid hormone, vitamin D3, and calcitonin. Fortunately, the renal tubule efficiently retrieves these and other constituents of the ultrafiltrate. Figure 42-1 illustrates the percentages of various filtered substances that remain in the tubule fluid at different points along the tubule. One hundred percent of the filtered glucose is reabsorbed by the proximal tubule; by the time the final urine is formed in the terminal collecting duct, approximately 99% of the filtered water and sodium has been retrieved. where Ucreatinine and Pcreatinine are the urinary and plasma concentrations of creatinine. By multiplying FEX by 100, the fractional excretion rate is expressed as the percentage of filtered X that is excreted. The fractional excretion rate, typically of sodium, can be used to assess the functional integrity of the renal tubules in clinical cases of acute renal failure. The structure of the proximal tubule and its proximity to the peritubular capillary facilitate the movement of tubule fluid components into the blood through two pathways: the transcellular pathway and the paracellular pathway. The tubule fluid flows over the apical surface of the proximal tubule epithelial cell. Substances transported through the transcellular pathway cross the apical plasma membrane, cytoplasm, and the basolateral plasma membrane into the interstitial fluid. Movement across the apical and basolateral plasma membranes occurs largely by carrier-mediated transport. The vast plasma membrane surface area of the proximal tubule contributes to transcellular transport. The apical plasma membrane has extensive microprojections, called microvilli, which collectively create the brush border (Figures 42-2 and 42-3). On the blood side of the cell, the basolateral plasma membrane has complex infoldings that enhance the surface area; the basolateral surface area equals that of the apical surface area in portions of the proximal tubule. The benefits of the enhanced plasma membrane surface area include increased capacity for the multitude of solute transporters and increased exposure to the luminal and interstitial fluids. The second route of transport in the proximal tubule is the paracellular pathway. Substances pass through the paracellular pathway from the tubule fluid across the zonula occludens, a permeable structure that attaches the proximal tubule cells to each other at the junction of the apical and basolateral plasma membrane domains (Figure 42-4). Paracellular transport occurs by passive diffusion or by solvent drag, which is the entrainment of solute by the flow of water. Substances crossing the zonula occludens enter the lateral intercellular space, which is thought to communicate freely with the interstitial fluid; from there, reabsorbed substances can be taken up by the peritubular capillary. Movement of water and solute from the interstitial fluid into the bloodstream is driven by Starling’s forces (see Chapter 23) and aided by the proximity of the peritubular capillary. In mammals the peritubular capillary originates at the glomerular efferent arteriole, subdivides, and wraps closely around the basal aspect of the proximal tubule (Figure 42-5). The plasma leaving the glomerulus has a high oncotic pressure because water and salts are filtered, but proteins are retained in the capillary. The peritubular capillary has low resistance, and thus the hydrostatic pressure in the capillary is low. Both these conditions—high peritubular plasma oncotic pressure and low peritubular capillary hydrostatic pressure—favor fluid and solute uptake from the interstitium into the bloodstream. Reabsorption of solutes takes place by a number of mechanisms, including primary active transport, carrier-mediated secondary active transport, solvent drag, and passive diffusion. (Transport mechanisms are described in Chapter 1.) In the proximal tubule, most solute reabsorption is driven by the active transport of sodium ions (Na+) by the sodium-potassium–adenosinetriphosphatase (Na+,K+-ATPase) pump, which is located in the basolateral plasma membrane. The Na+,K+-ATPase extrudes 3 Na+ ions and takes up 2 K+ ions on each turnover of the pump (Figure 42-6). Na+,K+-ATPase activity reduces the intracellular Na+ concentration and increases the intracellular K+ concentration. Outward diffusion of K+ down its chemical gradient through K+ channels makes the cell interior electrically negative relative to the exterior. These two factors create an electrochemical gradient for Na+ across the apical plasma membrane, favoring Na+ uptake from the tubule fluid into the cell. Na+ uptake across the apical plasma membrane is facilitated by specific transporters in the membrane that couple the movement of other solutes either in the same direction as Na+ (co-transport) or in the opposite direction (counter-transport). Specific Na+-dependent transporters for glucose (SGLT1, SGLT2), amino acids (EAAT3, SIT1, and more), phosphate (NaPi2a, NaPi2c, PiT-1), sulfate (NaS1), and citrate (NaDC1, NaDC3) mediate their uptake from the proximal tubule fluid by this mechanism of secondary active transport. The uptake of these substances increases their intracellular concentration, and they move across the basolateral plasma membrane and into the blood down their electrical or chemical gradient, facilitated by solute specific transporters and partly by passive diffusion. The list of solute transporters in the apical and basolateral plasma membranes continues to grow as more are discovered by researchers. Several of the apical Na+-coupled solute co-transporters and corresponding basolateral exit mechanisms are illustrated in Figure 42-6. Bicarbonate (HCO3−) reabsorption in the proximal tubule is also driven by the Na+ gradient, although indirectly. The chemical gradient for Na+ drives Na+ and proton (hydrogen ion, H+) counter-transport across the apical plasma membrane through a Na+/H+ exchanger (NHE3). Secreted H+ combines with filtered HCO3− in the tubule fluid to form water (H2O) and carbon dioxide (CO2), catalyzed by the enzyme carbonic anhydrase in the apical plasma membrane of proximal tubule cells. CO2 enters the cell across the apical plasma membrane, in part facilitated by the integral membrane protein, aquaporin 1 (AQP1). Cytoplasmic carbonic anhydrase catalyzes the hydroxylation of CO2 with OH− donated from H2O, forming H+ and HCO3− in the cell. HCO3− crosses the basolateral plasma membrane through a Na+,3-(HCO3−) co-transporter (NBCe1) and a Na+-dependent HCO3−/Cl− exchanger. The majority of H+ is transported into the tubule fluid through the Na+/H+ antiporter (NHE3); the electrogenic proton pump, H+ATPase, also contributes to proton secretion. By this complex mechanism, illustrated in Figure 42-7, the proximal tubule reabsorbs 60% to 85% of filtered HCO3−. Low–molecular-weight proteins are avidly reabsorbed by the proximal tubule, but by a different mechanism. Filtered proteins such as insulin, glucagon, parathyroid hormone, and many more are taken up at the apical plasma membrane by receptor-mediated endocytosis (see Figure 42-4). The proteins bind receptors (megalin and cubilin) in the plasma membrane, are endocytosed, and delivered by the endocytic vesicles to intracellular organelles called lysosomes while the receptors are recycled to the apical plasma membrane (Figure 42-8). Proteolytic enzymes in the lysosomes degrade the reabsorbed proteins; the amino acids that are the end products are transported into the interstitial fluid and returned to the blood. Diseased glomeruli often leak protein into the filtrate; in these instances, the proximal tubule endocytic machinery is upregulated and the lysosomal compartment is expanded, often to the extent that the increased number and size of lysosomes in proximal tubules are appreciable in histologic sections.
Solute Reabsorption
The Renal Tubule Reabsorbs Filtered Substances
Renal Tubule Function May Be Assessed by Determining Fractional Excretion Rate
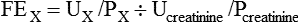
The Proximal Tubule Reabsorbs the Bulk of Filtered Solutes
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Solute Reabsorption
Only gold members can continue reading. Log In or Register to continue