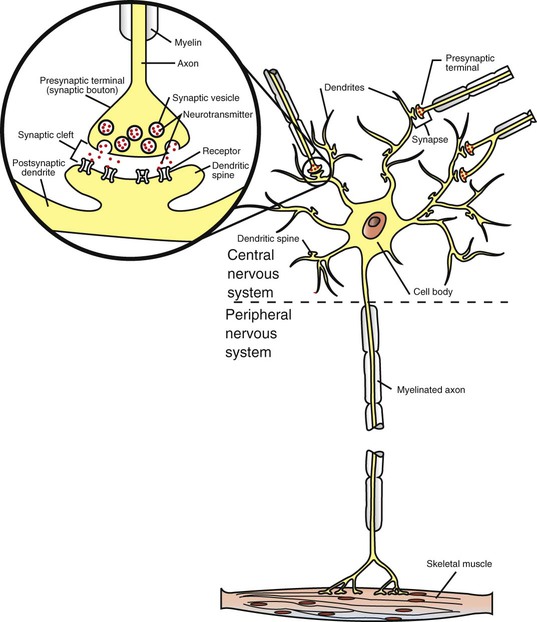
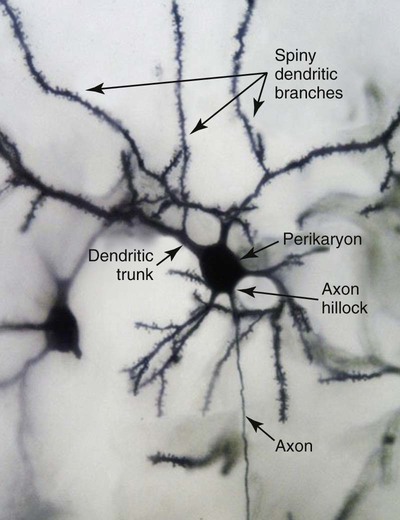
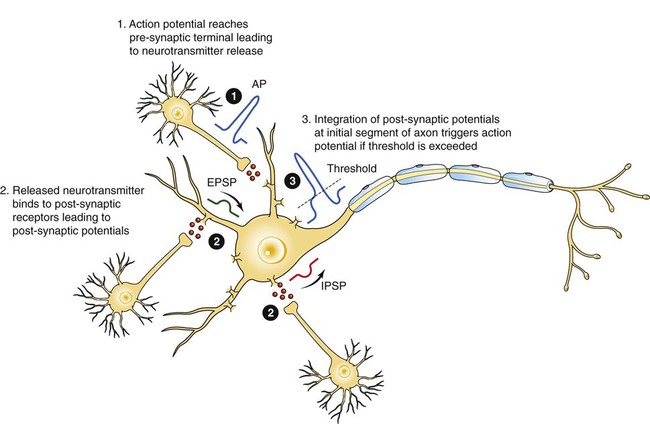
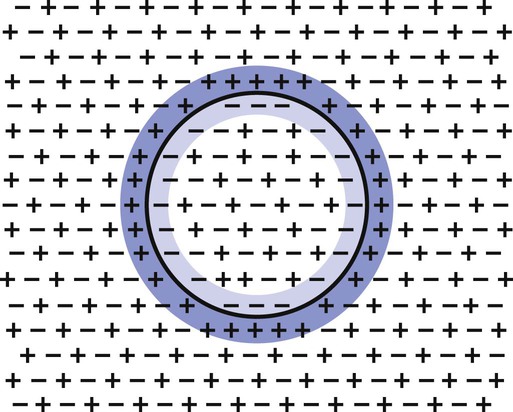
1. Neurons have four distinct anatomical regions. 2. Neuronal membranes contain a resting electrical membrane potential. 3. The resting membrane potential is the result of three major determinants. 4. The resting membrane potential can be changed by synaptic signals from a presynaptic cell. 5. Action potentials begin at the axon’s initial segment and spread down the entire length of the axon. There are two major classes of cells in the nervous system: the neuron and the glial cell (see Chapter 3). The neuron is the basic functional unit of the nervous system. The large number of neurons and their interconnections account for the complexity of the nervous system. The number of neurons in the vertebrate nervous system ranges greatly. There are approximately 100 million in a small mammal (e.g., mouse); 100 billion in a human; and more than 200 billion in whales and elephants: far more neurons in a nervous system than people on Earth, and there are 10 to 50 times more glial cells. The structural and functional support provided to neurons by glial cells and their potential to modulate neural communication make an important contribution to the operational integrity of the nervous system. The numbers of cells in the nervous system are huge, but knowing that they have common elements makes it easier to understand them. A typical neuron has four morphologically defined regions (Figure 4-1): the dendrites, the cell body, the axon, and the presynaptic terminals of the axon. These four anatomical regions are important in the major electrical and chemical responsibilities of neurons: receiving signals from the presynaptic terminals of other neurons (on dendrites), integrating these often-opposing signals (on the initial segment of the axon), transmitting action potential impulses along the axon, and signaling an adjacent cell from the presynaptic terminal. These functions are collectively analogous to the general role of the nervous system: collecting information from the environment, integrating that information, and producing an output that can change the environment. Axons branch near their ends into several specialized endings called presynaptic terminals (or synaptic boutons). When the action potential rapidly arrives, these presynaptic terminals transmit a chemical signal to an adjacent cell. The site of contact of the presynaptic terminal with the adjacent cell is called the synapse, shown in the inset in Figure 4-1. It is formed by the presynaptic terminal of one cell (presynaptic cell), the receptive surface of the adjacent cell (postsynaptic cell), and the space between these two cells (the synaptic cleft). Presynaptic terminals contain chemical transmitter–filled synaptic vesicles that can release their contents into the synaptic cleft. The presynaptic terminals of an axon usually contact the receptive surface of an adjacent neuron or muscle cell, usually on the neuron’s dendrites, but sometimes this contact is made on the cell body or, occasionally, on the presynaptic terminals of another cell (e.g., for presynaptic inhibition). On many neurons, presynaptic terminals often synapse on small protrusions of the dendritic membrane called dendritic spines (Figure 4-2 and see Chapter 5). The receptive surface of the postsynaptic cell contains specialized receptors for the chemical transmitter released from the presynaptic terminal. The signaling functions of the morphological components of the neuron can be briefly summarized as follows (Figure 4-3). Receptors, usually dendritic, receive neurochemical signals released from the presynaptic terminals of many other neurons. These neurochemical signals, after being transduced by the receptors into a different form (small voltage changes), are integrated at the initial segment of the axon. Depending on the results of this integration, an action potential (large voltage change) may be generated on the axon. The action potential travels very rapidly to the axon’s often distant presynaptic terminals to induce the release of chemical neurotransmitter onto another neuron or muscle cell. The origins of the resting electrical membrane potential are complicated, particularly in a quantitative way. In qualitative terms, however, the resting membrane potential is the result of the differential separation of charged ions, especially sodium (Na+) and potassium (K+), across the membrane and the resting membrane’s differential permeability to these ions as they attempt to move back down their concentration and electrical gradients (see Chapter 1). Even though the net concentration of positive and negative charges is similar in both the intracellular and extracellular fluids, an excess of positive charges accumulates immediately outside the cell membrane, and an excess of negative charges accumulates immediately inside the cell membrane (Figure 4-4). This makes the inside of the cell negatively charged with respect to the outside of the cell. The magnitude of the resulting electrical difference (or voltage) across the membrane varies from cell to cell, ranging from about 40 to 90 millivolts (mV), and is usually about 70 mV in mammalian neurons. Because the extracellular fluid is arbitrarily considered to be 0 mV, the resting membrane potential is –70 mV, more negative on the inside than on the outside. Three major factors cause the resting membrane potential. • The Na+, K+ pump. Cell membranes have an energy-dependent pump that pumps Na+ ions out of the cell and draws K+ ions into the cell against their concentration gradients. This maintains the differential distribution of each of these charged ion species across the membrane that underlies their ability to produce a voltage across the membrane. The pump itself makes a small, direct contribution to the resting membrane potential because it pushes three molecules of Na+ out for every two molecules of K+ drawn into the cell, thus concentrating positive charges outside the cell. • An ion species will move toward a dynamic equilibrium if it can flow across the membrane. Using K+ as an example, the concentration difference across the membrane actively maintained by the Na+, K+ pump produces a concentration gradient, or chemical driving force, that attempts to push the ion passively across the membrane from high concentration inside the cell toward low concentration outside. If K+ can flow across ion channels in the membrane, exiting K+ leaves behind unopposed negative charge (often from negatively charged protein macromolecules trapped inside the cell) that builds an electrical gradient, or electrical driving force, pulling K+ back inside the cell. These opposing gradients eventually produce a dynamic equilibrium, even though there may still be more K+ inside than outside, as well as a charge imbalance across the membrane. This uneven distribution of charge at dynamic equilibrium produces a voltage across the membrane called the equilibrium potential for that ion. When an ion species can flow across a channel in the membrane, it flows toward its equilibrium state, and it drives the voltage across the membrane toward its equilibrium potential. • Differential permeability of the membrane to diffusion of ions. The resting membrane is much more permeable to K+ than to Na+ ions because there are vastly more K+ leak channels than Na+ leak channels in the membrane. This greater membrane permeability to K+ means that K+ ions can more closely approach their dynamic equilibrium state, and equilibrium potential, compared with Na+ ions, which have difficulty moving across the membrane. Therefore the equilibrium potential for the more permeant K+ ions (about –90 mV in many mammalian neurons) will have the predominant influence on the value of the resting membrane potential compared with the equilibrium potential of the vastly less permeant Na+ ions (about +70 mV in many mammalian neurons). Therefore, as noted earlier, the resting membrane potential of many mammalian neurons is about –70 mV, close to the equilibrium potential for K+.
The Neuron
Neurons Have Four Distinct Anatomical Regions
Neuronal Membranes Contain a Resting Electrical Membrane Potential
The Resting Membrane Potential Is the Result of Three Major Determinants
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


The Neuron
Only gold members can continue reading. Log In or Register to continue