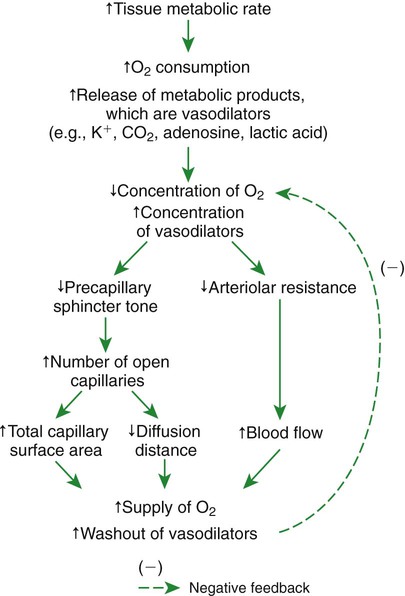
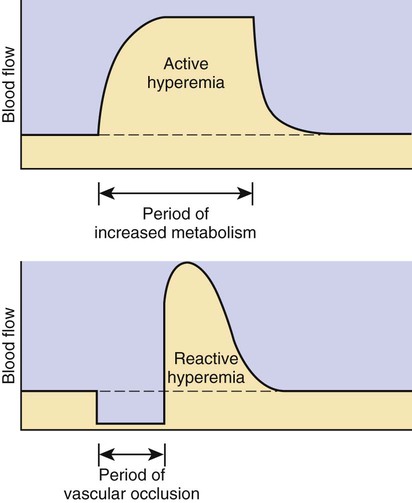
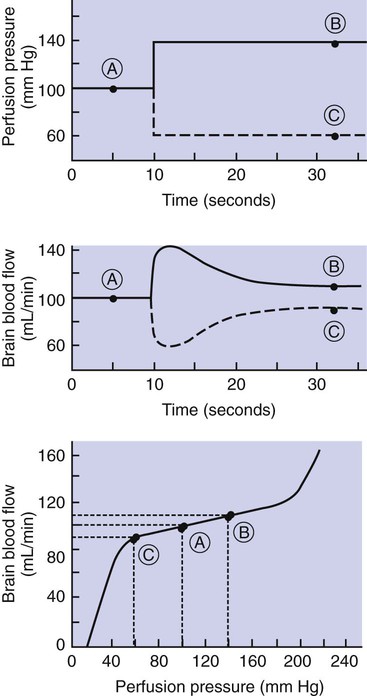
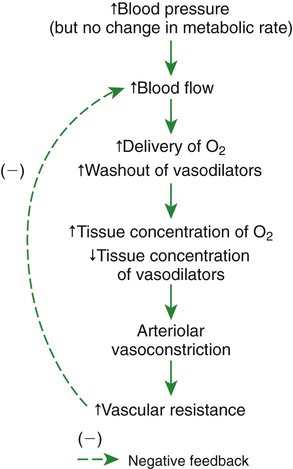
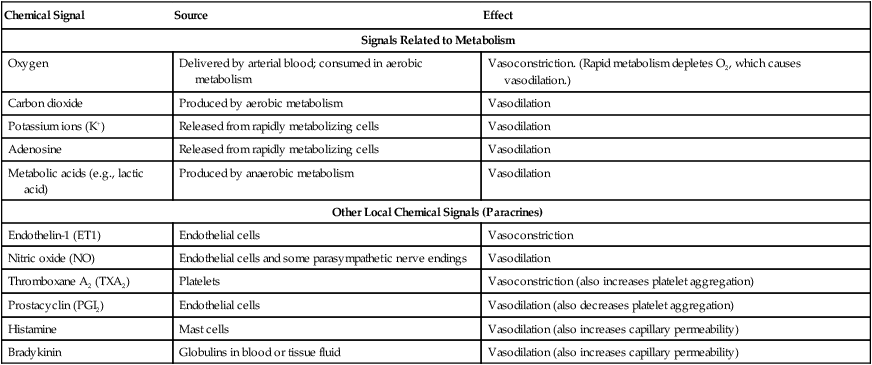
1. Vascular resistance is affected by intrinsic and extrinsic control mechanisms. 2. Metabolic control of blood flow is a local mechanism that matches the blood flow of a tissue to its metabolic rate. 3. Autoregulation is a relative constancy of blood flow in an organ despite changes in perfusion pressure. 4. Many chemical signals act locally (as paracrines) to exert important control on vascular resistance. 5. Regardless of the status of arterioles, mechanical compression can reduce blood flow to a tissue. As described in Chapter 22, the blood flow through any organ or tissue is determined by the perfusion pressure (arterial pressure minus venous pressure) and by the resistance of the blood vessels of the organ (and by no other factors), as follows: In general, the factors that affect arteriolar resistance can be divided into intrinsic and extrinsic factors. Extrinsic control involves mechanisms that act from outside an organ or tissue, through nerves or hormones, to alter arteriolar resistance. Intrinsic control is exerted by local mechanisms within an organ or tissue. For example, as described in Chapter 23, histamine is released from mast cells of a tissue in response to injury or during an allergic reaction. Histamine acts locally on the arteriolar smooth muscle to relax it. Dilation of the arterioles decreases arteriolar resistance and therefore increases blood flow to the tissue. Histamine is an example of a paracrine: a substance released from one type of cell that acts on another cell type in the vicinity. Paracrine signaling molecules move by diffusion, which is why paracrine signaling is only effective over very short distances. A second example of intrinsic control is the arteriolar dilation and increased blood flow during exercise in skeletal muscle. This example illustrates the general phenomenon of metabolic control of blood flow: tissues tend to increase their blood flow whenever their metabolic rate increases. Metabolic control of blood flow works by means of chemical changes within the tissue. When the metabolic rate of a tissue increases, its consumption of oxygen increases, and there is an increased rate of production of metabolic products, including carbon dioxide, adenosine, and lactic acid. Also, some potassium ions (K+) escape from rapidly metabolizing cells, and these ions accumulate in the interstitial fluid. Therefore, as the metabolism of a tissue increases, the interstitial concentration of oxygen decreases, and the interstitial concentrations of metabolic products and K+ increase. All these changes have the same effect on arteriolar smooth muscle: they relax it (Table 24-1). The arterioles dilate, vascular resistance decreases, and more blood flows through the tissue. TABLE 24-1 Chemical Signals Important in Local Control of Systemic Arterioles* *Some of these chemical signals have different effects on pulmonary blood vessels than on systemic vessels. A high level of oxygen, for example, causes dilation of pulmonary vessels, whereas the effect in systemic vessels is vasoconstriction. See Chapter 46 for more details. Low levels of oxygen and high concentrations of metabolic products and K+ also cause relaxation of the precapillary sphincters (in the tissues that have them), and this opens more of the capillaries in the tissue to blood flow. As explained in Chapter 23, the opening of more capillaries decreases the diffusion distance between fresh, oxygenated blood and the metabolizing cells of the tissue. Opening more capillaries also increases the total capillary surface area for diffusional exchange. The net result of the increased blood flow, the decreased diffusion distance, and the increased total capillary surface area is a more rapid delivery of oxygen and other metabolic substrates to the tissue cells and a more rapid removal of metabolic waste products from the tissue. Metabolic control of blood flow involves negative feedback. The accumulation of metabolic products and the lack of oxygen initiate vasodilation, which increases blood flow. The increased blood flow removes the accumulating metabolic products and delivers additional oxygen. A new balance is reached when the increased blood flow closely matches the increased metabolic needs of the tissue. Figure 24-1 summarizes the major features of metabolic control of blood flow. The same metabolic control mechanisms that account for active hyperemia also explain reactive hyperemia. During the period when mechanical compression restricts blood flow, metabolism continues in the compressed tissue; metabolic products accumulate, and the local concentration of oxygen decreases. These metabolic effects cause dilation of the arterioles and a decrease in arteriolar resistance. When the mechanical obstruction to flow is removed, blood flow increases above normal until the “oxygen debt” is repaid and the excess metabolic products have been removed from the compressed tissue. Figure 24-2 compares active and reactive hyperemia. Figure 24-3 summarizes an experiment that demonstrates autoregulation in the brain. Initially, the perfusion pressure (arterial pressure minus venous pressure) in this animal is 100 mm Hg, and the blood flow to the brain is 100 milliliters per minute (mL/min) (point A). When perfusion pressure is increased suddenly to 140 mm Hg, brain blood flow rises initially to 140 mL/min but returns toward its initial level over the next 20 to 30 seconds. Eventually, blood flow reaches a stable level of about 110 mL/min (point B). Conversely, if the perfusion pressure is decreased suddenly from 100 to 60 mm Hg, blood flow in the brain decreases initially to 60 mL/min but returns toward its initial level over the next 20 to 30 seconds (see dashed lines in the top and middle graphs of Figure 24-3). Eventually, blood flow reaches a stable level of about 90 mL/min (point C). These stable responses are plotted in the bottom graph. The remainder of the bottom graph is obtained in a similar way; that is, perfusion pressure is set artificially to various levels, ranging from 40 to 220 mm Hg, and the resulting steady-state levels of blood flow are plotted. Figure 24-4 shows how the metabolic control mechanisms previously described can account for the phenomenon of autoregulation. If the metabolic rate of an organ does not change but perfusion pressure is increased above normal, the increased pressure forces additional blood flow through the organ. The additional blood flow accelerates the removal of metabolic products from the interstitial fluid and increases the rate of oxygen delivery to the interstitial fluid. Therefore the concentration of vasodilating metabolic products in the interstitial fluid decreases, and the concentration of oxygen in the interstitial fluid increases. These changes cause the arterioles of the tissue to constrict, which increases the resistance to blood flow above normal. The consequence is that blood flow decreases back toward its initial level, despite the continuation of the elevated perfusion pressure.
Local Control of Blood Flow
Vascular Resistance Is Affected by Intrinsic and Extrinsic Control Mechanisms
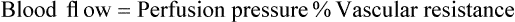
Metabolic Control of Blood Flow Is a Local Mechanism That Matches the Blood Flow of a Tissue to Its Metabolic Rate
Chemical Signal
Source
Effect
Signals Related to Metabolism
Oxygen
Delivered by arterial blood; consumed in aerobic metabolism
Vasoconstriction. (Rapid metabolism depletes O2, which causes vasodilation.)
Carbon dioxide
Produced by aerobic metabolism
Vasodilation
Potassium ions (K+)
Released from rapidly metabolizing cells
Vasodilation
Adenosine
Released from rapidly metabolizing cells
Vasodilation
Metabolic acids (e.g., lactic acid)
Produced by anaerobic metabolism
Vasodilation
Other Local Chemical Signals (Paracrines)
Endothelin-1 (ET1)
Endothelial cells
Vasoconstriction
Nitric oxide (NO)
Endothelial cells and some parasympathetic nerve endings
Vasodilation
Thromboxane A2 (TXA2)
Platelets
Vasoconstriction (also increases platelet aggregation)
Prostacyclin (PGI2)
Endothelial cells
Vasodilation (also decreases platelet aggregation)
Histamine
Mast cells
Vasodilation (also increases capillary permeability)
Bradykinin
Globulins in blood or tissue fluid
Vasodilation (also increases capillary permeability)
Autoregulation Is a Relative Constancy of Blood Flow in an Organ Despite Changes in Perfusion Pressure
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Local Control of Blood Flow
Only gold members can continue reading. Log In or Register to continue