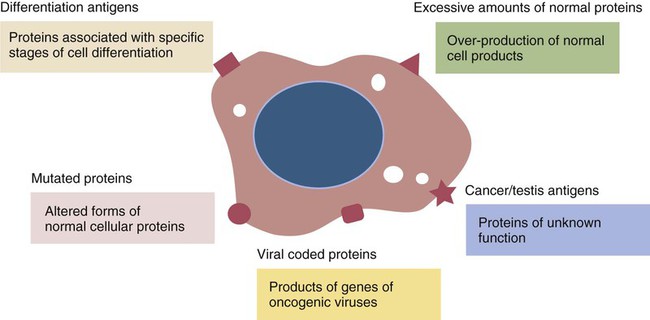
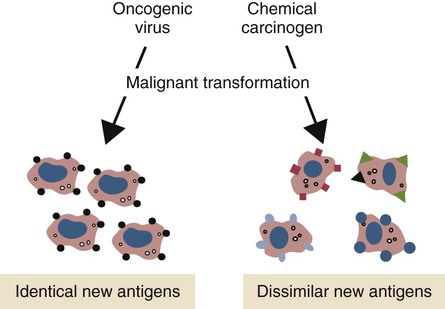
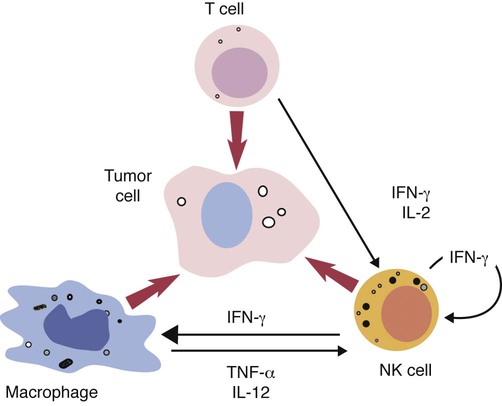
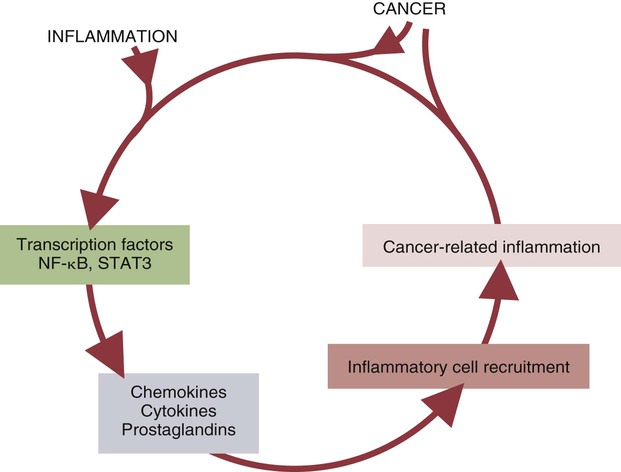
• Cancer cells may differ antigenically from normal body cells. As a result they may trigger an immune response. This immune response, however, may not be very strong. • Because cancer cells can mutate as they grow, those cells that survive may be selected for their lack of antigenicity. • The most important mechanism of destruction of spontaneous tumors probably involves killing by natural killer (NK) cells. NK cells recognize and attack target cells that fail to express major histocompatibility (MHC) class I molecules. • Under some circumstances, cytotoxic T cells, activated macrophages, or antibodies may also attack cancer cells. • Failure of antitumor immunity involves not only tumor cell selection but also the activities of regulatory T cells and blocking antibodies. • It has proved very difficult to devise effective, consistent antitumor immunotherapy. This may involve administration of cytokines or antibodies or active immunization using vaccines against tumor antigens. The immune surveillance theory soon ran into problems. Common human cancers such as those of the lung or breast do not develop more frequently in immunodeficient individuals. Likewise, nude (nu/nu) mice, although T cell deficient, are no more susceptible than normal mice to chemically induced or spontaneous tumors (Chapter 37). Finally, it became clear that many tumor antigens induce tolerance in a manner similar to normal self-antigens. Thus most evidence has failed to support the idea that the immune system distinguishes between tumor cells and normal, healthy cells. Second, there are mutated forms of normal cellular proteins. For example, melanoma cells may express the products of mutated oncogenes on their surface (Figure 33-1). Some tumor antigens are recognized because they are abnormally glycosylated. Chemically induced tumors may express mutated surface antigens unique to the tumor and not to the inducing chemical (Figure 33-2). Because carcinogenic chemicals can produce many different mutations, tumors induced by a single chemical in different animals may be antigenically different. Even within a single chemically induced tumor mass, antigenically distinct subpopulations of cells exist. As a result, immunity to one chemically induced tumor does not prevent growth of a second tumor induced by the same chemical. If tumor cells express unusual antigens, why are they not regarded as foreign and attacked by the immune system? The main problem appears to be that the abnormal molecules are not appropriately presented to the cells of the immune system, especially cytotoxic T cells. Nevertheless, on occasion, tumor cells may be attacked by NK cells, cytotoxic T cells, activated macrophages, or antibodies (Figure 33-3). It is likely that the most important of these attackers are NK cells. The tumor microenvironment plays a large part in determining the behavior of a tumor. The tumor cells communicate extensively with nearby cells. Among the most important of these are fibroblasts and inflammatory cells. As a result, the elimination of tumor cells by immune mechanisms is determined in part by the presence of inflammation. Inflammatory diseases increase the risk for developing many types of cancer; conversely, the use of nonsteroidal antiinflammatory drugs reduces tumor susceptibility (Figure 33-4). Mutations in oncogenes such as ras and myc are closely linked to the inflammatory pathways. Inflammatory cytokines, chemokines, and cells are present in the microenvironment of early tumors. Blocking of inflammatory mediators, key transcription factors, or inflammatory cells decreases the incidence and spread of cancer. Conversely, adoptive transfer of inflammatory cells may promote tumor development. More than half of the mass of a tumor may consist of supporting cells, including fibroblasts, macrophages, and vascular endothelial cells. Cancers cannot spread and metastasize without the support of these cells. The process by which these stromal cells are recruited is closely related to inflammation. Inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), and macrophages are often required for tumor development and spread. Cancer and inflammation are linked by two pathways. The intrinsic pathway is activated by mutations leading to neoplasia. These include the activation of various oncogenes, chromosomal rearrangements, or the inactivation of tumor suppressor genes. Cells transformed in this way generate transcription factors, produce inflammatory mediators, and generate an inflammatory microenvironment around tumor cells. The extrinsic pathway involves the development of inflammation by inflammatory or infectious disease. Toll-like receptor (TLR) ligands or interleukin-1β (IL-1β) generate transcription factors such as NF-κB by acting through the MyD88 pathway. These transcription factors may be generated not only in inflammatory cells but also in any nearby tumor or stromal cells. NF-κB is an important endogenous tumor promoter. It stimulates inflammatory cytokine production and promotes the survival of tumor cells by reducing expression of the antiapoptotic gene bcl-2 (Chapter 18). Natural killer (NK) cells are the subject of Chapter 19. These are cytotoxic cells that respond to abnormal or stressed cells without prior priming and belong to the innate immune system. They have two major types of receptors: inhibitory receptors that can recognize the presence of major histocompatibility complex (MHC) class I molecules on a target cell surface and in so doing are prevented from killing their targets; and activating receptors that can recognize the presence of certain stress-induced proteins on cell surfaces and as a result are activated and kill their targets. Thus NK cells effectively kill two types of cellular targets: cells that fail to express MHC class I molecules and cells that express certain stress-related proteins. Both conditions commonly apply to cancer cells. As a result, NK cells play a key role in the destruction of tumors. Some tumor-derived molecules may redirect macrophage activities so that they promote tumor growth. Thus tumor-derived IL-4, IL-6, IL-10, TGF-β, prostaglandin E2, and macrophage colony-stimulating factor can deactivate or suppress the activation of macrophages and Th1 responses. TGF-β can convert antitumor effector cells into Treg cells. Many tumors produce indolamine 2,3-dioxygenase (IDO), a potent immunosuppressive agent and a suppressor of NK cell function (Chapter 20).
Resistance to Tumors
Tumors as Allografts
Tumor Antigens
Immunity to Tumors
Inflammation and Tumors
Cellular Defenses
Natural Killer Cells
Failure of Immunity to Tumor Cells
Immunosuppression
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Resistance to Tumors
Only gold members can continue reading. Log In or Register to continue