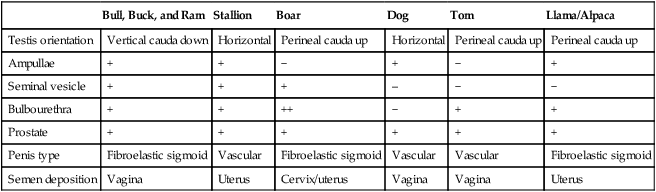
1. The male reproductive system consists of many individual organs acting in concert to produce spermatozoa and deliver them to the female’s reproductive tract. 2. Normal spermatogenesis requires maintenance of uniform testicular temperature 2° to 6° C lower than core body temperature. 3. Emission is the release of spermatozoa and accessory gland fluids into the pelvic urethra, whereas ejaculation is the forceful expulsion of semen from the urethra. 1. Spermatogenesis is a lengthy orchestrated process in which diploid stem cells divide by mitosis to maintain their own numbers and cyclically produce progeny that undergo meiotic division and differentiation into haploid germ cells. 2. Testicular size can predict daily sperm production. Hypothalamic-pituitary-testicular axis 1. The reproductive system of the male is regulated by the hypothalamus, which is hormonally linked to the anterior pituitary and testes by luteinizing hormone and follicle-stimulating hormone. 1. Puberty is not synonymous with sexual maturity. 2. Puberty results from a continuous process of endocrine changes that are initiated shortly after birth. 1. Anabolic steroids are androgen derivatives that exert negative feedback on the hypothalamic-pituitary-testicular axis. The male reproductive system is made up of a number of individual organs acting in concert to produce spermatozoa and deliver them to the reproductive tract of the female. This concerted effort involves both the neuroendocrine (hypothalamus and anterior pituitary glands) and the genital system. The genital organs consist of two testes, each suspended within the scrotum by a spermatic cord and external cremaster muscle; two epididymides; two deferent ducts; accessory sex glands; and the penis. The accessory sex glands include paired ampullae, paired seminal vesicles (vesicular glands), a prostate gland, and paired bulbourethral glands (Cowper glands). The presence of individual accessory glands, the testicular orientation, the type of penis, and the site of semen deposition in the female are dependent on the species (Table 40-1). TABLE 40-1 Emission is the release of spermatozoa and accessory gland fluids into the pelvic urethra as a result of sympathetically mediated thoracolumbar reflex contraction of the smooth muscle in the ductus deferens and accessory glands. Ejaculation is the forceful expulsion of semen from the urethra and is prompted by a parasympathetically mediated sacral reflex that induces rhythmic contractions of the bulbospongiosus, ischiocavernosus, and urethralis muscles. After ejaculation, a sacral sympathetically mediated increase in the smooth muscle tone of the cavernous spaces increases the outflow of blood, and contraction of the retractor penis muscle withdraws the penis into the prepuce. The seminal characteristics of the different species are listed in Table 40-2. TABLE 40-2 Seminal Characteristics from Domestic Animals
Reproductive Physiology of the Male
Functional Anatomy
The Male Reproductive System Consists of Many Individual Organs Acting in Concert to Produce Spermatozoa and Deliver Them to the Female’s Reproductive Tract
Bull, Buck, and Ram
Stallion
Boar
Dog
Tom
Llama/Alpaca
Testis orientation
Vertical cauda down
Horizontal
Perineal cauda up
Horizontal
Perineal cauda up
Perineal cauda up
Ampullae
+
+
−
+
−
+
Seminal vesicle
+
+
+
–
−
−
Bulbourethra
+
+
++
−
+
+
Prostate
+
+
+
+
+
+
Penis type
Fibroelastic sigmoid
Vascular
Fibroelastic sigmoid
Vascular
Vascular
Fibroelastic sigmoid
Semen deposition
Vagina
Uterus
Cervix/uterus
Vagina
Vagina
Uterus
Emission Is the Release of Spermatozoa and Accessory Gland Fluids into the Pelvic Urethra, Whereas Ejaculation Is the Forceful Expulsion of Semen from the Urethra
Parameter
Bull
Ram
Buck
Boar
Stallion
Alpaca/Llama
Dog*
Tom
Ejaculate volume (mL)
5-8
0.7-1.3
0.7-1.4
150-250
50-100
0.7-3.0
2.0-25
0.03-0.3
Spermatozoa concentration (millions/mL)
800-2,000
2,000-3,500
2,000-4,500
200-300
150-300
80-250
60-500
1,700-2,900
Motile spermatozoa (%)
40-75
60-80
60-85
50-80
40-75
40-70
50-90
40-90
Normal spermatozoa (%)
65-95
80-95
75-95
70-90
60-90
55-85
50-90
50-90 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Reproductive Physiology of the Male
Only gold members can continue reading. Log In or Register to continue
