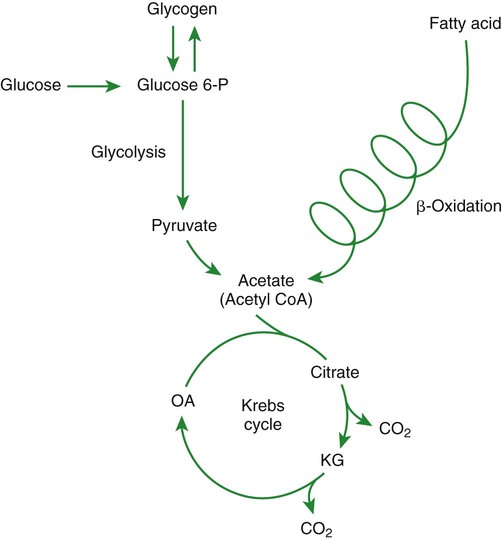
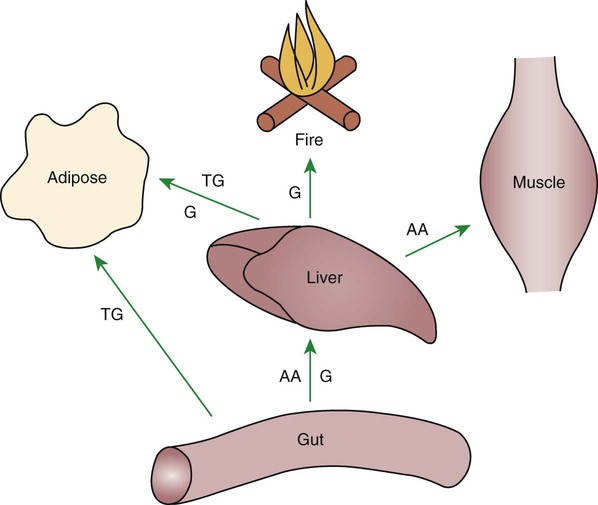
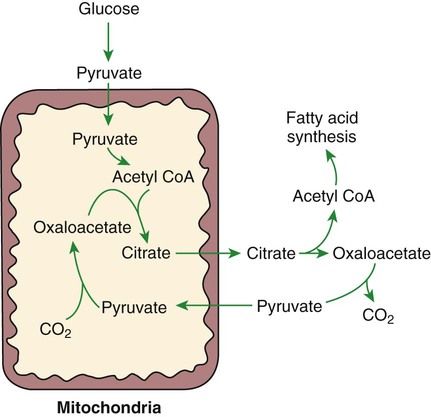
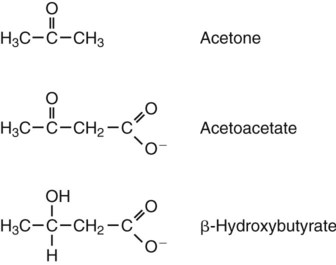
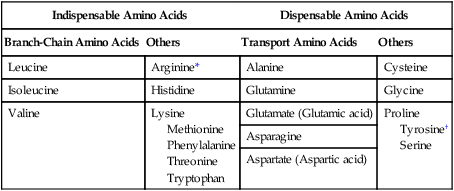
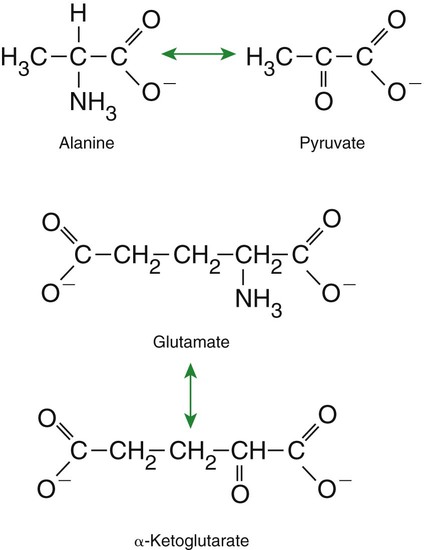
1. Homeostatic mechanisms balance the supply and demand of almost all nutrients. 1. The tricarboxylic acid (or Krebs) cycle is the major energy-yielding pathway of fuel utilization in the body. 1. The major metabolic fuels consist of glucose, amino acids, fatty acids, and ketone bodies. 2. Glucose is the central fuel in the energy metabolism of most animals. 3. Amino acids are important fuels in addition to being the building blocks of protein. 4. Fatty acids are the major form of energy storage in the animal body. 5. Ketone bodies are fat-derived, water-soluble metabolites that serve as glucose substitutes. Nutrient utilization during the absorptive phase 1. During the absorptive phase, the liver takes up glucose and converts it into glycogen and triglyceride. 2. The conversion of glucose to fatty acids is an irreversible process. 3. Transport of fatty acids out of the liver is through chylomicron-like particles known as very-low-density lipoproteins. 4. Amino acids can be classified into groups on the basis of metabolic characteristics. 5. Amino acids are extensively modified during absorption. 6. Many amino acids are removed by the liver on “first pass,” never reaching the systemic circulation. 7. Some amino acids taken up by the liver are used for protein synthesis. 8. Most amino acids taken up by the liver are converted to carbohydrates. 9. Not all amino acids are subject to hepatic destruction. 10. Metabolism at the tissue level is coordinated with hepatic metabolism and results in the deposition of fuel into storage tissues during the absorptive period. 11. Insulin promotes the synthesis of protein and the deposition of glycogen in muscle. 12. Insulin-stimulated uptake of amino acids by muscle results in a net increase in muscle protein synthesis. 13. During the absorptive phase, triglyceride accumulation in adipose tissue occurs by two mechanisms: uptake from very-low-density lipoproteins and direct lipid synthesis from glucose. Nutrient utilization during the postabsorptive phase 1. Hepatic metabolism switches from glucose utilization to glucose production during the postabsorptive phase. 2. Fuel mobilization in peripheral tissues occurs when the blood insulin concentration declines. 3. Muscle reacts to a metabolic demand for glucose by mobilizing amino acids to support hepatic gluconeogenesis. 4. Muscle release of amino acids is related to reduced glucose and amino acid uptake. 5. The complex pattern of muscle amino acid catabolism and release is necessary to accommodate the liver’s limited capacity for uptake of branch-chain amino acids and to facilitate the removal of amino nitrogen from the muscle. 6. The reaction of adipose tissue during the postabsorptive phase is to mobilize fatty acids. Nutrient utilization during prolonged energy malnutrition or complete food deprivation 1. During prolonged periods of fasting or undernutrition, glucose and amino acids are conserved by extensive utilization of fats and ketone bodies for energy production. 2. A large portion of the fatty acids released from adipose tissue is taken up directly by the liver. 3. Hepatic ketone body formation is promoted by low glucose availability, a high glucagon/insulin ratio, and a ready supply of fatty acids. 4. Glucagon plays an important role in the excessive production of ketone bodies in diabetes mellitus. 5. Fatty acids cannot be used for glucose synthesis. 6. Ketone bodies are formed in the mitochondria from acetyl coenzyme A. 7. Hepatic very-low-density lipoproteins may be synthesized from adipose-derived fatty acid as well as from newly synthesized fatty acid. 8. Hormonal conditions direct the distribution of very-low-density lipoprotein fatty acids in the body. 9. Changes in growth hormone concentrations may aid in shifting peripheral fuel utilization from glucose and amino acids to ketone bodies and fatty acids. Special fuel considerations of ruminants 1. Ruminants exist in a perpetual state of gluconeogenesis because of their unique digestive process. Energy-supplying nutrients are referred to as metabolic fuels, and the physiological mechanisms for maintaining the supply of fuels and matching it to demand constitute fuel homeostasis. Fuel homeostasis is maintained by several mechanisms: the insulin-glucagon axis, the hypothalamic-pituitary axis, and the central nervous system (CNS). This chapter discusses some of the ways in which fuel is stored during the absorptive period of digestion and subsequently mobilized when needed to supply energy requirements. You may want to review Chapter 1 and the section on insulin and glucagon in Chapter 34 before reading this chapter. The Krebs cycle is the pathway through which all body fuels are eventually “burned.” In this cycle carbon compounds from the various body fuels are completely oxidized to carbon dioxide and water. Much of the energy released in this process is captured as ATP, the direct source of chemical energy for most physiological processes. The substrate for oxidation in the Krebs cycle is acetate, a two-carbon compound that enters the Krebs cycle in an activated form known as acetyl-CoA. Figure 32-1 illustrates that glucose and fatty acids are the sources of acetate for oxidation. Selecting between these two sources of acetate is a major function of fuel homeostasis. The first step through which glucose is used as a fuel is through glycolysis, the series of biochemical steps that initiate the oxidation of glucose. Through glycolysis, each molecule of glucose is converted to two molecules of pyruvate, a key molecule that stands at the junction of several metabolic pathways. When glucose is completely oxidized for fuel, pyruvate is converted to acetate (acetyl CoA) and passes into the Krebs cycle, the site of its complete oxidation. For the study of fuel homeostasis, you should appreciate that the conversion of glucose to pyruvate is reversible. Therefore any metabolic pathway that can lead to the production of pyruvate can also lead to the creation of glucose. Figure 32-1 illustrates a key relationship between pyruvate, acetate, and oxaloacetic acid. The conversion of pyruvate to acetate is irreversible while the conversion of pyruvate to oxaloacetate can flow in either direction. Thus any metabolic pathway that can lead to the creation of pyruvate or oxaloacetate can lead to the synthesis of glucose. Creation of glucose through such pathways is called gluconeogenesis. The process of gluconeogenesis occurs in the liver and to a small extent also in the kidneys. It occurs in no other tissues. Another pathway for glucose oxidation is the pentose-phosphate pathway. This is a quantitatively minor pathway that does not have great impact on fuel homeostasis. However, it is an important metabolic pathway in erythrocytes (RBCs), which have an absolute need for glucose, although these cells’ overall need for energy is small compared with the rest of the body. Fatty acids are stored in adipose tissue in the form of triglycerides (also called triacylglycerols), which consist of three fatty acid molecules linked to a glycerol molecule by ester bonds (see Figure 30-24). Triglycerides are an ideal form of energy storage for animals. They are highly reduced molecules (there is little oxygen compared with the amount of carbon and hydrogen), which means they are a concentrated energy source, having more than twice the caloric value per gram than carbohydrates or amino acids. In addition, adipose tissue contains little water compared with protein or glycogen, the storage forms of the other two potential fuels. Thus, adipose tissue is undiluted by bulky water, allowing it to be a concentrated form of energy storage that permits animals to carry with them a maximal amount of energy at a minimal amount of weight. Fats, however, have a metabolic disadvantage; they are not water soluble. Therefore, special transport systems are needed to enable fats to be distributed among the tissues through the blood and lymph systems. In addition, fatty acids cannot be converted to glucose, so they cannot, under usual circumstances, contribute to the energy supply of the CNS. However, fatty acids can be converted to ketone bodies. In monogastric species, ketone bodies are formed exclusively in the liver and are used by a wide variety of tissues. Some tissues, including cardiac muscle, use ketone bodies instead of glucose. In ruminants the ketone body β-hydroxybutyrate is formed from butyrate in the rumen epithelium. Thus, in ruminants, ketone bodies are not only products of fatty acid metabolism, but also products of normal digestion. Elevated serum concentrations of ketone bodies are characteristic of several diseases associated with abnormalities of fuel homeostasis. This fact might lead one to conclude that ketone bodies are abnormal, or even toxic, metabolites. In fact, when present in physiological concentrations, ketone bodies are important fuels that occupy an integral part of the scheme of fuel homeostasis. Figure 32-2 illustrates the chemical structure of the three major ketone bodies. During the absorptive phase as nutrient absorption takes place, metabolic events in the liver and peripheral organs are coordinated to direct nutrients into storage molecules and storage sites. Figure 32-3 illustrates the general scheme of metabolism during the absorptive phase. When a meal is ingested, insulin secretion begins even before maximal absorption of glucose is achieved. This secretion is stimulated by the action of gastric inhibitory peptide (see Chapter 27) and perhaps other enteric hormones. Early insulin secretion ensures that the liver and other tissues will be “primed” and ready for the arrival of glucose from the gut. A large portion of the glucose absorbed postprandially is taken up by the liver as portal blood traverses the hepatic sinusoids. Under the influence of insulin, glucose in the liver is directed into glycogen synthesis. The net effect is that glucose from the digestion and absorption of carbohydrate is stored in the liver during absorptive periods. This process moderates the flow of glucose from the gut to the general circulation and keeps blood glucose concentrations from becoming excessively high during the digestion of a carbohydrate meal. Insulin exerts its stimulatory effect on hepatic glycogen synthesis by stimulating intracellular metabolic pathways that lead to the formation of glycogen. These effects are discussed further in reference to the counterbalancing effects of glucagon. The synthesis of fatty acids from glucose begins with glycolysis. This pathway leads to the production of two pyruvate molecules for each molecule of glucose consumed. Pyruvate can then enter the mitochondria to be activated to acetyl coenzyme A (acetyl CoA) for entry into the Krebs cycle, as discussed previously. However, the Krebs cycle is for energy generation, and during the absorptive period, there is more than enough acetyl CoA and Krebs cycle activity to provide for energy needs; therefore the excess acetyl CoA must be shunted away from the Krebs cycle. The excess acetyl CoA combines with oxaloacetate to form citrate in what is essentially the first reaction of the Krebs cycle. Instead of continuing through the Krebs cycle reactions, however, during the absorptive period much of the citrate is transported out of the mitochondria into the cytosol. When in the cytosol, each citrate molecule contributes two carbons toward the synthesis of fatty acids. The remaining portion of the citrate molecule cycles back into the mitochondria for further use. Citrate serves as a carrier molecule to transport two-carbon units out of the mitochondria because acetyl CoA cannot pass the mitochondrial membrane directly (Figure 32-4). When formed in the liver, fatty acids must be transported either to adipose tissue for storage or to other tissues (e.g., muscle) for direct utilization for energy production. Because fatty acids are insoluble in blood, some special transport mechanism for their distribution is necessary. This mechanism is through the hepatic formation of triglyceride-rich serum lipoproteins, also known as very-low-density lipoproteins (VLDLs); these triglyceride-rich lipoproteins are much less dense than other lipoproteins in blood serum. In the synthesis of VLDLs, fatty acids are first esterified to form triglycerides, and the triglycerides are wrapped in a coat of phospholipid, cholesterol, and specific proteins (Figure 32-5). This is essentially the same mechanism by which fatty acids are transported out of the enterocytes after absorption from the gut. In the latter case the lipoproteins are called chylomicrons. The VLDLs of the liver are smaller than chylomicrons but have a similar structure and function. The mechanisms by which VLDLs and chylomicrons deliver fatty acids to peripheral tissues are further discussed in relation to peripheral tissues. The discussion of amino acid absorption and metabolism becomes complicated because not all amino acids are subject to the same reactions. For this discussion the amino acids are divided into two groups, each containing two subgroups (Table 32-1). The major groups are the “nutritionally dispensable” amino acids and the “nutritionally indispensable” amino acids. Within the dispensable amino acid group, glutamate, aspartate, alanine, glutamine, and asparagine are separated out as transport amino acids; within the indispensable amino acid group, leucine, isoleucine, and valine form a special subgroup known as the branch-chain amino acids (BCAAs). The transport amino acids are utilized in several reactions in which amino groups are transferred from molecule to molecule or organ to organ. TABLE 32-1 Metabolic Classification of Amino Acids *Indispensable for cats, but not required in the diets of many other species. The profile of amino acids in the portal vein is considerably different from that of the diet, indicating that amino acid destruction and transformation occur during the absorptive process. Essentially all the glutamate and much of the aspartate in the diet are removed by the intestinal epithelial cells during absorption, so the portal blood is almost devoid of glutamate and contains little aspartate. Much of the nitrogen from glutamate and aspartate is transferred to pyruvate to form the amino acid alanine, which is present in high concentrations in portal blood. The metabolism of the transport amino acids in the intestinal epithelium is a good example of both the way in which amino groups can be gained and lost and how the metabolism of amino acids interfaces with the metabolism of carbohydrate. Glutamate and aspartate are similar to two Krebs cycle intermediates, α-ketoglutarate and oxaloacetate, differing only by the presence of an amino group or a keto-oxygen. Carbohydrates and amino acids having this relationship are said to be analogues; thus, α-ketoglutarate is the keto-analogue of glutamate, and pyruvate is the keto-analogue of alanine (Figure 32-6). All amino acids can form keto-analogues, and all keto-analogues can be readily converted back to their parent amino acids.
Postabsorptive Nutrient Utilization
Homeostatic Mechanisms Balance the Supply and Demand of Almost All Nutrients
The Furnace
The Tricarboxylic Acid (or Krebs) Cycle Is the Major Energy-Yielding Pathway of Fuel Utilization in the Body
The Fuels
The Major Metabolic Fuels Consist of Glucose, Amino Acids, Fatty Acids, and Ketone Bodies
Glucose Is the Central Fuel in the Energy Metabolism of Most Animals
Fatty Acids Are the Major Form of Energy Storage in the Animal Body
Ketone Bodies Are Fat-Derived, Water-Soluble Metabolites That Serve as Glucose Substitutes
Nutrient Utilization during the Absorptive Phase
During the Absorptive Phase, the Liver Takes up Glucose and Converts It into Glycogen and Triglyceride
The Conversion of Glucose to Fatty Acids Is an Irreversible Process
Transport of Fatty Acids out of the Liver Is Through Chylomicron-Like Particles Known as Very-Low-Density Lipoproteins
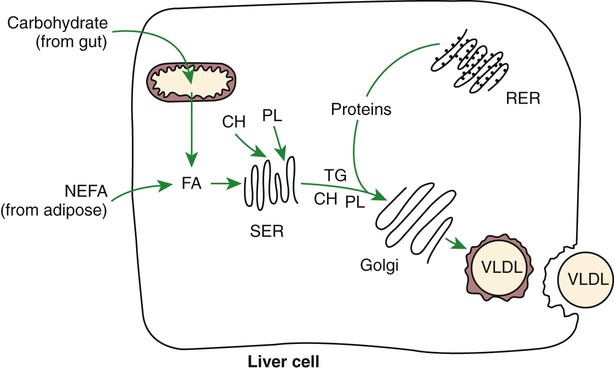
Amino Acids Can Be Classified into Groups on the Basis of Metabolic Characteristics
Indispensable Amino Acids
Dispensable Amino Acids
Branch-Chain Amino Acids
Others
Transport Amino Acids
Others
Leucine
Arginine*
Alanine
Cysteine
Isoleucine
Histidine
Glutamine
Glycine
Valine
Lysine
Methionine
Phenylalanine
Threonine
Tryptophan
Glutamate (Glutamic acid)
Proline
Tyrosine†
Serine
Asparagine
Aspartate (Aspartic acid)
Amino Acids Are Extensively Modified During Absorption
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Postabsorptive Nutrient Utilization
Only gold members can continue reading. Log In or Register to continue