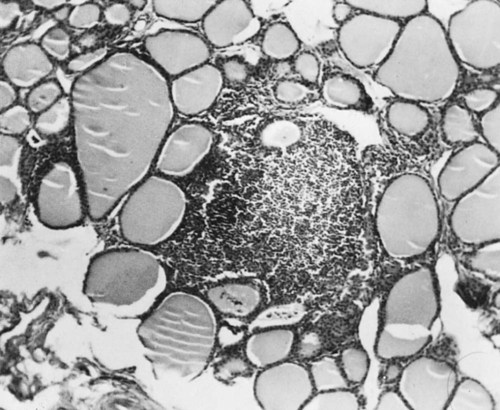
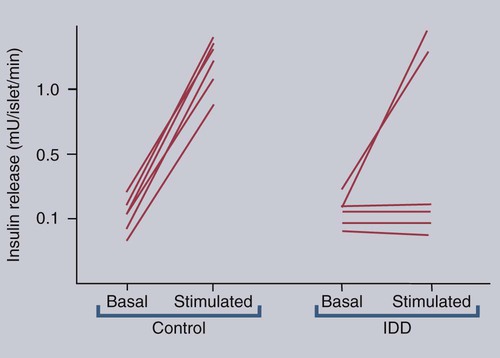
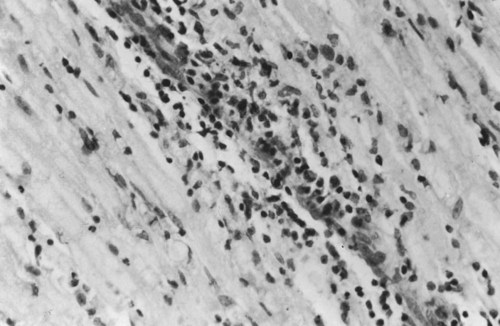
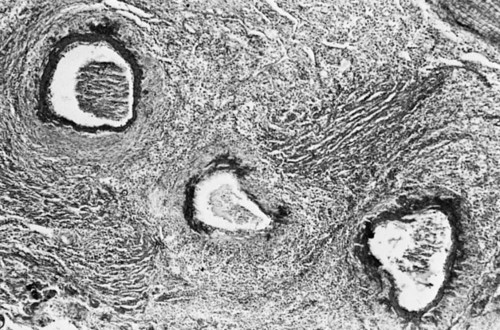
• Domestic mammals suffer from a diverse array of autoimmune diseases. Any organ or tissue is a potential victim of autoimmune attack. • The most common autoimmune diseases in domestic mammals involve the endocrine system, the skin, and blood cells. • Treatment of autoimmune diseases usually involves suppression of the destructive inflammatory lesion by corticosteroids. These may be supplemented by the use of other immunosuppressive drugs. Dogs, humans, and chickens suffer from autoimmune thyroiditis as a result of the production of autoantibodies against thyroglobulin or thyroid peroxidase. These antibodies may also react with triiodothyronine (T3) or thyroxine (T4). Affected dogs may also show a delayed skin reaction to intradermally injected thyroid extract, suggesting that cell-mediated immunity contributes to the disease. Several dog breeds are predisposed to thyroiditis, and relatives of affected animals may have antithyroid antibodies although clinically normal. A familial form of hypothyroidism has been demonstrated in Beagles and Great Danes. Dogs from high-risk breeds such as Dobermans tend to develop the disease when young, whereas dogs from low-risk breeds tend to develop it when older. Unfortunately, by the time the disease is diagnosed, the dog may already have been bred. Affected thyroids are infiltrated with plasma cells and lymphocytes, and germinal center formation may occur (Figure 35-1). The invading lymphocytes probably cause epithelial cell destruction through antibody-dependent cell-mediated cytotoxicity (ADCC) and T cell cytotoxicity. In dogs, clinical signs appear after about 75% of the thyroid is destroyed. These signs are those of hypothyroidism; that is, the animals are fat and inactive and show patchy hair loss. The most common problems are a dry, dull, coarse coat; scaling; hypotrichosis; slow hair regrowth; hyperpigmentation; myxedema; and pyoderma. Other signs include myopathy, hyperlipidemia, hypothermia, anestrus, galactorrhea, diarrhea or constipation, and polyneuropathy. Tests of thyroid function such as a radioimmunoassay for plasma T4 or T3 only confirm the existence of hypothyroidism. A thyroid-stimulating hormone (TSH) response test is more useful because it can confirm the inability of the affected thyroid to respond to TSH. (Plasma T4 levels are measured before and after injection of TSH.) In order to confirm autoimmune thyroiditis, a biopsy must show the characteristic lymphocytic infiltration. Antithyroid antibodies must be detected in serum using an enzyme-linked immunosorbent assay (ELISA), immunoblot, or an indirect fluorescent antibody test (Chapter 41). There is little correlation between antithyroid antibody titers and disease severity. Management of affected animals involves replacement therapy with sodium levothyroxine (synthetic T4). Improvement should be seen within 4 to 6 weeks. There is no cure for this disease, and the success of treatment depends on effective replacement therapy. In humans, insulin-dependent diabetes mellitus (IDDM) is an autoimmune disease mediated by autoantibodies against an islet cell enzyme called glutamic acid decarboxylase. Some cases of IDDM in dogs may also be immunologically mediated. The canine disease is associated with pancreatic islet atrophy and a loss of β cells. In some cases, the islets are infiltrated by lymphocytes. Experimentally, circulating mononuclear cells from diabetic dogs have suppressed insulin production by cultured mouse islet cells. Serum from IDDM dogs lysed these islet cells in the presence of complement (Figure 35-2). When dog serum was tested for antibodies against cultured β cells by immunofluorescence, 9 of 23 diabetic dogs showed strongly positive reactions, and an additional 3 showed a weak reaction. Only 1 of 15 normal dogs gave a positive response. Thus cytotoxic cells or antibodies or both may be responsible for β cell destruction in dogs. A familial predisposition to IDDM has been observed in Samoyeds. Genes that may influence the development of canine diabetes mellitus include those for the regulatory cytokines interferon-γ (IFN-γ), interleukin-12 (IL-12), IL-4, and IL-10. These candidate genes were screened for single nucleotide polymorphisms (SNPs) in multiple different dog breeds. Significant associations were observed for IL-4 in Collies, Cairn Terriers, and Schnauzers and for IL-10 in the Cavalier King Charles Spaniel. This suggests that cytokine genes that influence the Th1/Th2 balance may determine susceptibility to diabetes. If sciatic nerve tissue is used to immunize experimental dogs, it provokes experimental allergic neuritis. After a latent period of 6 to 14 days, the animals develop an ascending polyneuritis and gradual paralysis (Figure 35-3). The disease is due to peripheral nerve demyelination resulting from autoimmune attack. The CSF in acute cases of SRMA contains high IgA and CXCL8 levels and mature neutrophils. Serum IgA and acute-phase proteins (C-reactive protein and α2-macroglobulin) are also elevated. Analysis of a patient’s T cells indicates that production of IL-2 and IFN-γ are depressed, whereas Th2 production of IL-4 is enhanced and probably accounts for the increased IgA production. About 30% of these dogs have a positive lupus erythematosus (LE) cell test but no detectable antinuclear antibody activity (Chapter 36). In chronic cases the CSF contains predominantly mononuclear cells. On necropsy, the spinal meningeal arteries show fibrinoid degeneration, intimal or medial necrosis, and hyalinization and are infiltrated with lymphocytes, plasma cells, and macrophages, and a few neutrophils (Figure 35-4). Complete obliteration of the blood vessel lumina may occur, whereas rupture and thrombosis of inflamed vessels may lead to hemorrhage, compression, and infarction.
Organ-Specific Autoimmune Diseases
Autoimmune Endocrine Disease
Lymphocytic Thyroiditis
Insulin-Dependent Diabetes Mellitus
Autoimmune Neurological Disease
Canine Polyneuritis
Steroid-Responsive Meningitis-Arteritis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree