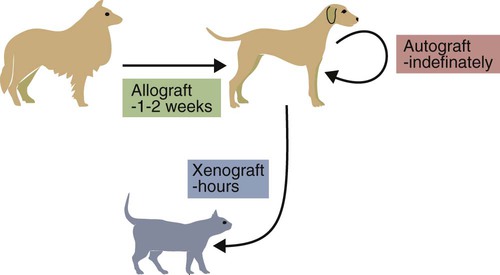
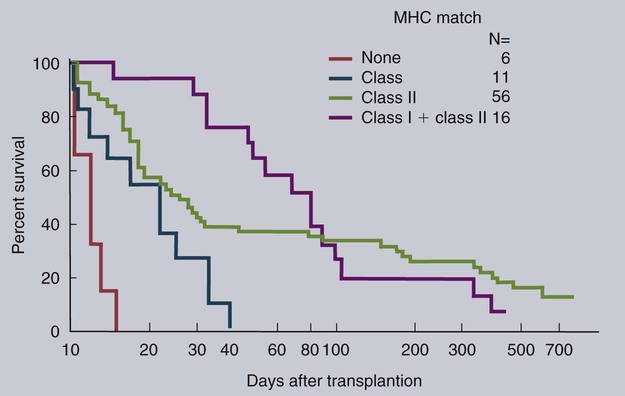
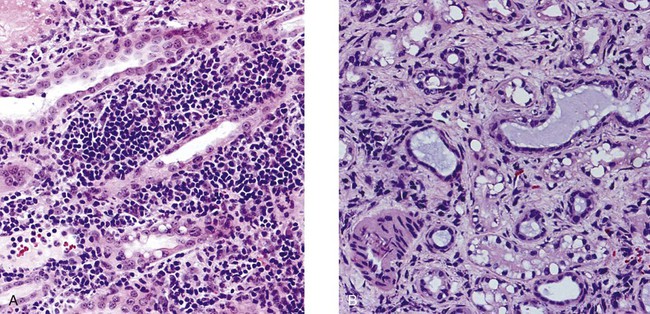
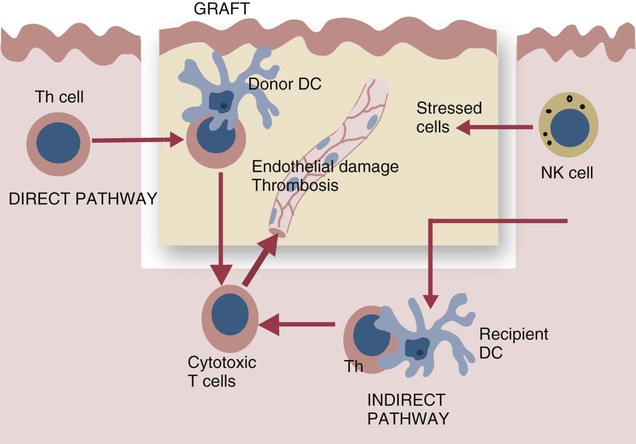
• Organ grafts between two unrelated individuals of the same species are called allografts. • Allografts are rejected by the recipient as a result of immune responses directed against donor blood group antigens and histocompatibility antigens. • The response to donor histocompatibility antigens causes acute rejection and is mainly mediated by cytotoxic T cells attacking graft vascular endothelium. • Chronic rejection and rejection directed against donor blood groups are mainly antibody mediated. • Bone marrow stem cell allografts given to immunosuppressed recipients can attack the recipient and cause graft-versus-host disease. • Some allografts, such as those from the cornea, are not readily rejected. • The fetus can be considered an allograft but is not rejected as a result of multiple immunosuppressive mechanisms acting at the maternal-placental interface. Advances in surgery have permitted the transfer of many tissues or organs between different parts of the body or between different individuals. When moved to a different part of an animal’s own body, such transplants do not trigger an immune response. This type of graft within an individual is called an autograft (Figure 32-1). Examples of autografting include the use of skin to cover a burn in plastic surgery and the use of a segment of vein to bypass blocked cardiac arteries. Since autografts do not express foreign antigens, they do not trigger an immune response. When an organ is transplanted into a genetically dissimilar animal, the recipient will mount an immune response against many different antigens in and on the cells of the allograft. These are called histocompatibility antigens. Three types of histocompatibility antigens are of major importance in stimulating graft rejection. These are the MHC class I molecules, the MHC class II molecules, and the major blood group molecules. All are expressed on the surface of the graft cells, but their distribution varies. MHC class I antigens are found on almost all nucleated cells. The major blood group antigens are found both on red cells and nucleated cells. MHC class II antigens, in contrast, have a restricted distribution that varies among mammals (Chapter 11). For example, in rats and mice, MHC class II molecules are expressed only on the professional antigen-presenting cells (APCs): macrophages, dendritic cells, and B cells. In other species, such as humans and pigs, MHC class II molecules are also expressed on the endothelium of renal arteries and glomeruli, the sites where host cells first make contact with the graft. These MHC class II molecules are recognized as foreign and trigger the rejection process. It is interesting to note that, as a result of these differences, it is much easier to prolong renal allograft survival in laboratory rodents than in humans or pigs. As would be expected, grafts that differ minimally from the recipient will generally survive longer than grafts that are highly incompatible. When blood group A-O-compatible pigs are given renal allografts, median survival is about 12 days for MHC-unmatched grafts, 25 days for grafts compatible for MHC class I alone, 32 days for grafts compatible for MHC class II alone, and 80 days for grafts compatible for both class I and class II (Figure 32-2). When dogs are given MHC-unmatched renal allografts, the grafts survive for about 10 days. Completely matched allografts in dogs survive for about 40 days. A more impressive result is obtained with canine liver grafts, which survive for about 8 days in unmatched animals and for 200 to 300 days in DLA-matched recipients. During the acute rejection process, the grafted tissue gradually becomes infiltrated with cytotoxic T cells, which cause progressive damage to the endothelial cells lining small blood vessels (Figure 32-3). The T cells roll along the endothelial surface and bind using leukocyte function-associated antigen-1 (LFA-1). T cell–mediated damage releases chemokines that attract more T cells into the graft. Cellular destruction, stoppage of blood flow, hemorrhage, and death of the grafted organ follow thrombosis of these vessels. The blood vessels of second organ grafts become blocked even more rapidly as a result of the action of antibodies and complement on the vascular endothelium. This secondary reaction is specific for any graft from the original donor or from a donor syngeneic with the first. It is not restricted to any particular site or to any specific organ since MHC and blood group molecules are present on most nucleated cells. In practice it is usually not difficult to ensure that the donor and recipient have identical major blood group antigens. MHC compatibility is much harder to achieve because MHC polymorphism ensures that individuals differ widely in their MHC haplotype. In general, the more closely donor and recipient are related, the less will be their MHC difference. For this reason it is preferable that grafts be obtained from a recipient’s parents or siblings. If this is not possible, a donor must be selected at random and the inevitable rejection responses suppressed by drugs such as cyclosporine or tacrolimus (Chapter 39). The allograft rejection process is directed against the dominant antigens on the cells of the graft. The MHC molecules tend to trigger a T cell–mediated rejection response, whereas the blood group antigens tend to trigger antibody formation. The rejection process may be divided into two stages. First, the host’s lymphocytes encounter the antigens of the graft and trigger a response. Second, cytotoxic T cells and antibodies from the host enter the graft and destroy graft cells (Figure 32-4). Although cytotoxic T cells are of major importance in acute allograft rejection, B cells, eosinophils, and macrophages also play a significant role in hyperacute and chronic rejection (Figure 32-5). Hyperacute rejection occurs when the recipient has preexisting antibodies to graft MHC or blood groups. These bind to graft vascular endothelial cells, activate complement by the classical pathway, and cause endothelial cell lysis. The damaged endothelial cells trigger platelet deposition as well as multiple chemokines and cytokines, especially IL-1β, CXCL8, and CCL2. These attract leukocytes as well, and the damage results in thrombosis and infarction. Anti-MHC antibodies also play a major role in secondary rejection, in which they activate the classical complement pathway and mediate antibody-dependent cytotoxic cell activity.
Organ Graft Rejection
Grafting of Organs
Allograft Rejection
Histocompatibility Antigens
Renal Allografts
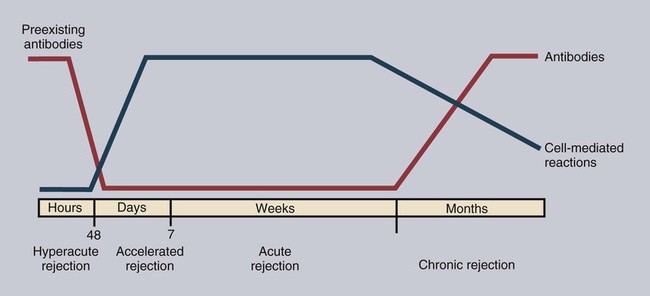
Clinical Allograft Rejection
Pathogenesis of Allograft Rejection
Adaptive Mechanisms
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Organ Graft Rejection
Only gold members can continue reading. Log In or Register to continue