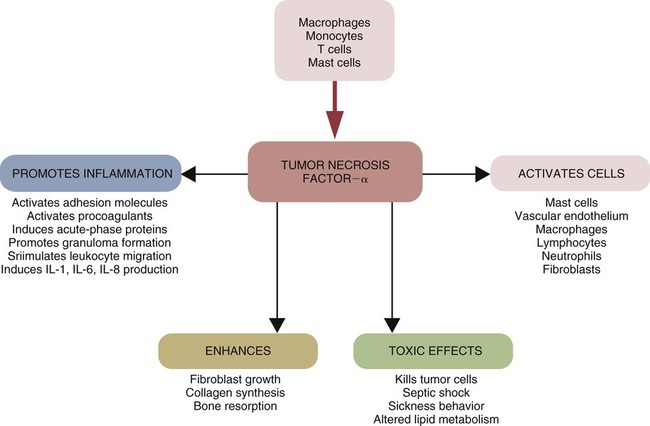
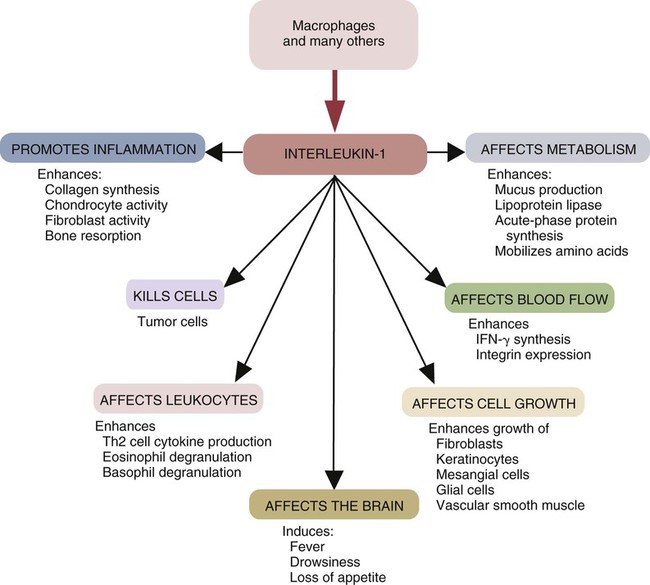
• Three major proinflammatory cytokines produced by sentinel cells are tumor necrosis factor-α, interleukin-1, and interleukin-6. • Stimulation of toll-like receptors (TLRs) and other pattern-recognition receptors (PRRs) activates the sentinel cells and trigger secretion of these cytokines. • Sentinel cells and damaged cells produce many other molecules that trigger and maintain inflammation. • Some inflammatory molecules are secreted by nerves. • Collectively, these molecules trigger local increases in blood flow, attract defensive cells such as neutrophils, promote increased vascular permeability leading to tissue swelling, and kill invading microorganisms. When exposed to infectious agents or their PAMPs, sentinel cell signaling pathways activate the genes that result in the synthesis and secretion of three major cytokines. These are, tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-6. TNF-α is produced very early in inflammation, and this is followed by waves of IL-1 and then by IL-6. Activated sentinel cells also secrete a large number of small chemotactic proteins called chemokines. These chemokines attract defensive cells to sites of microbial invasion. At the same time, stimulated sentinel cells synthesize enzymes such as nitric oxide synthase 2 (NOS2) that in turn generates oxidants such as nitric oxide (NO). They also make the enzyme cyclooxygenase-2 (COX-2) that generates inflammatory lipids such as the prostaglandins and leukotrienes. When produced in sufficient quantities to reach the brain and liver, these molecules cause a fever and sickness behavior and promote an acute-phase response (Chapter 6). If the sentinel cells detect the presence of damaged or foreign DNA or RNA, such as that from viruses, they will also secrete the antiviral type I interferons, IFN-α and IFN-β (Chapter 26). TNF-α is a protein of 17 Da produced by sentinel cells in response to TLR stimulation. TNF-α can also be produced by stimulated endothelial cells, T cells, B cells, and fibroblasts. It is produced in both soluble or membrane-bound forms. The membrane-bound form is cleaved from the cell surface by a protease called TNF-α convertase. The soluble TNF-α released in this way triggers the release of chemokines and cytokines from nearby cells and promotes the adherence, migration, attraction, and activation of leukocytes (Figure 3-1). Later, TNF-α facilitates the transition from innate to adaptive immunity by enhancing antigen presentation and T cell activation. TNF-α production is stimulated not only through the toll-like receptors (TLRs) but also by molecules secreted by nerves such as the neurotransmitter neurokinin-1. TNF-α is an essential mediator of inflammation because in combination with IL-1 it triggers changes in the vascular endothelial cells that line small blood vessels. A local increase in TNF-α causes the classic signs of inflammation, including heat, swelling, pain, and redness. Circulating TNF-α can depress cardiac output, induce microvascular thrombosis, and cause capillary leakage. TNF-α acts on neutrophils (key defensive cells in inflammation; see Chapter 4) to enhance their ability to kill microbes. It attracts neutrophils to sites of tissue damage and increases their adherence to vascular endothelium. It stimulates macrophage phagocytosis and oxidant production. It amplifies and prolongs inflammation by promoting macrophage synthesis of other mediators such as NOX2 and COX-2, and it also activates mast cells. TNF-α induces macrophages to increase its own synthesis together with that of IL-1. As its name implies, TNF-α can kill some tumor cells and virus-infected cells. In high doses, TNF-α may cause septic shock (Chapter 6). There are two TNF receptors: TNFR1 is found on most cells, where it can bind both soluble and cell-associated TNF; and TNFR2 is restricted to the cells of the immune system and responds only to cell-associated TNF. Based on sequence, functional, and structural similarities, cytokines may be grouped into families. For example, the TNF family consists of multiple proteins, several of which share the ability to kill cells. Other important members include TNF-β (or lymphotoxin) (Chapter 18); CD40L (Chapter 14); FasL (CD95L) (Chapter 18); and TRAIL (Chapter 19). When stimulated through CD14 and TLR4, sentinel cells such as macrophages also produce IL-1α and IL-1β. IL-1β is produced as a large precursor protein that is cleaved by caspase-1 to form the active 17.5-kDa molecule. Ten- to 50-fold more IL-1β is produced than IL-1α, and whereas IL-1β is secreted, IL-1α remains attached to the cell. Therefore, IL-1α only acts on cells in direct contact with the macrophage (Figure 3-2). Transcription of IL-1β messenger RNA (mRNA) occurs within 15 minutes of ligand binding. It reaches a peak 3 to 4 hours later and levels off for several hours before declining. Like TNF-α, IL-1β acts on nearby cells to initiate and amplify inflammation. Thus it acts on vascular endothelial cells to make them adhesive for neutrophils. IL-1 also acts on other macrophages to stimulate their synthesis of NOS2 and COX-2. During severe infections, IL-1β circulates in the bloodstream where, in association with TNF-α, it is responsible for sickness behavior. Thus it acts on the brain to cause fever, lethargy, malaise, and lack of appetite. It acts on muscle cells to mobilize amino acids, causing pain and fatigue. It acts on liver cells to induce the production of new proteins, called acute-phase proteins, that assist in the defense of the body (Chapter 6). The most important IL-1 receptors are CD121a and CD121b. CD121a is a signaling receptor, whereas CD121b is not. CD121b thus inhibits IL-1 functions. Soluble CD121b can bind IL-1 and acts as an IL-1 antagonist. IL-1 activity is also regulated by IL-1 receptor antagonist (IL-1RA), an inactive molecule that binds and blocks CD121a. IL-1RA is therefore an important regulator of IL-1 activity and inflammation. It reduces mortality in septic shock and graft-versus-host disease and has antiinflammatory effects (Chapter 6). IL-1 is a member of a family of cytokines that regulate innate immune responses. Other family members include IL-1RA, IL-18, IL-33, IL-36, IL-37, and possibly IL-38 (Chapter 8 and Appendix 3). All these cytokines signal through a group of closely related receptors. IL-1 and IL-18 are produced as precursor proteins that require activation by inflammatory caspases. Other members of the IL-1 family that play a role in innate immunity include IL-36, which has a proinflammatory effect, and IL-37, which has an antiinflammatory effect. IL-6 is a 22- to 28-kDa glycoprotein produced by macrophages, T cells, and mast cells. Its production is triggered by bacterial endotoxins, IL-1, and TNF-α. IL-6 affects both inflammation and adaptive immunity. It promotes some aspects of inflammation, especially in response to tissue damage and severe infections since it is a major mediator of the acute-phase reaction and of septic shock (Chapter 6). It is an important mediator in antibacterial resistance. It has been suggested that IL-6 regulates the transition from a neutrophil-dominated process early in inflammation to a macrophage-dominated process later. It is also produced by muscles during exercise. IL-6 also has an antiinflammatory role in that it inhibits some activities of TNF-α and IL-1 and promotes the production of IL-1RA as well as a suppressive cytokine called IL-10 (Chapter 20). The IL-6 receptor is a heterodimer consisting of two proteins, gp130 and IL-6R, found on T cells, neutrophils, macrophages, hepatocytes, and neurons. Chemokines are a family of at least 50 small (8- to 10-kDa) chemotactic cytokines. They coordinate the migration of cells and hence dictate the course of many inflammatory and immune responses (Table 3-1). Chemokines are produced by sentinel cells, including macrophages and mast cells. They are classified into four families on the basis of their amino acid sequences (Figure 3-3). For example, the CC, or α, chemokines have two contiguous cysteine (C) residues, whereas the CXC, or β, chemokines have two cysteine residues separated by another amino acid (X). (Chemokine nomenclature is based on this classification, each molecule or receptor receiving a numerical designation. Ligands have the suffix “L” [e.g., CXCL8], whereas receptors have the suffix “R” [e.g., CXCR1].) Table 3-1 Nomenclature of Some Selected Chemokines and Their Receptors
Innate Immunity
Proinflammatory and Antimicrobial Mediators
Products of Sentinel Cells
Cytokines
Tumor Necrosis Factor-α
Interleukin-1
Interleukin-6
Chemokines
CURRENT NAME
OLD NAME
RECEPTOR
α Family
CCL2
MCP-1
CCR2
CCL3
MIP-1α
CCR1, CCR5
CCL4
MIP-1β
CCR5
CCL5
RANTES
CCR1, CCR3, CCR5
CCL7
MCP-3
CCR3
CCL8
MCP-2
CCR3
CCL11
Eotaxin
CCR3
CCL13
MCP-4
CCR3
CCL20
MIP-3α
CCR6
CCL22
MDC
CCR4
CCL26
Eotaxin 3
CCR3
CCL28
MEC
CCR3
β Family
CXCL1
GRO1
CXCR2
CXCL7
MDGF
CXCR2
CXCL8
IL-8
CXCR1, CXCR2
CXCL12
SDF
CXCR4
CXCL13
BCA-1
CXCR5
γ Family
XCL1
Lymphotactin
XCR1
δ Family
CX3CL1
Fractalkine
CX3CR1 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Innate Immunity: Proinflammatory and Antimicrobial Mediators
Only gold members can continue reading. Log In or Register to continue