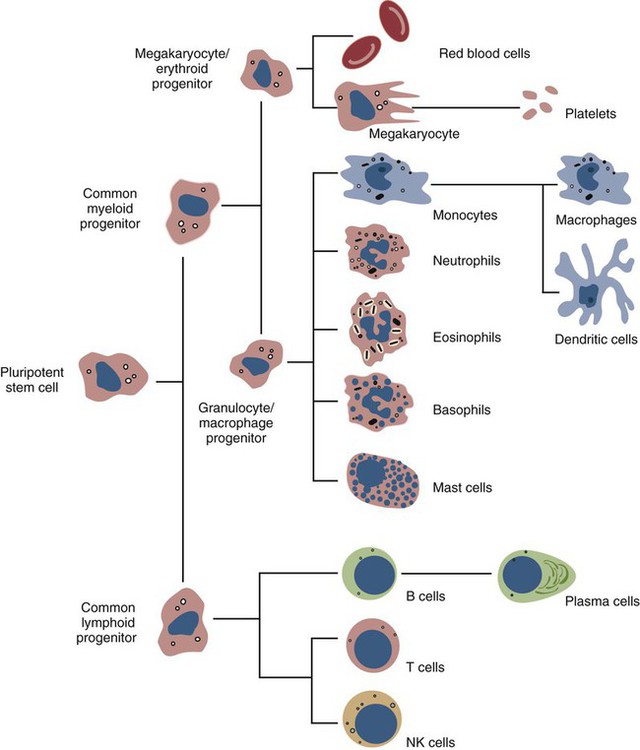
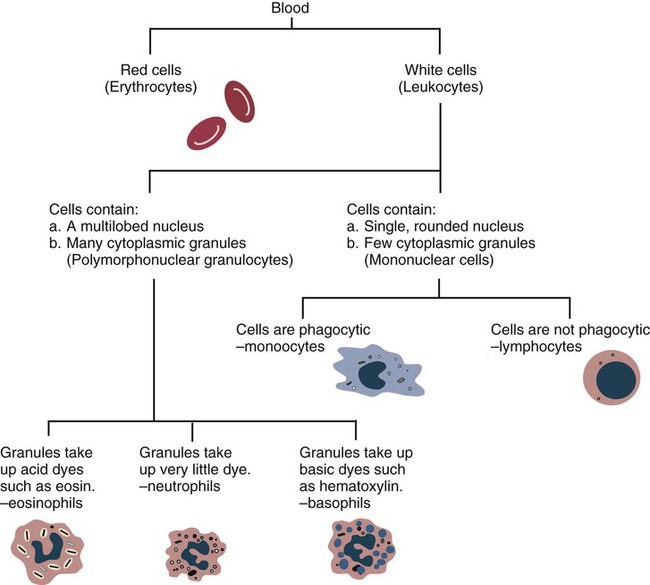
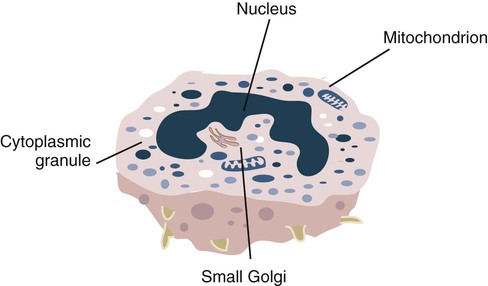
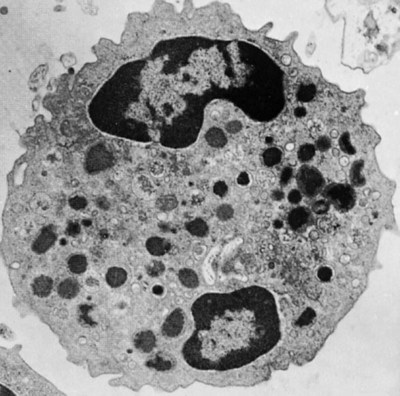
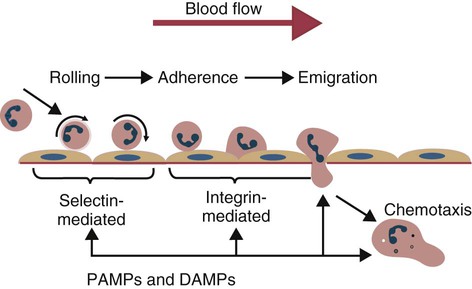
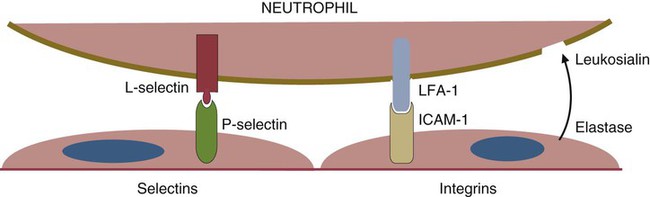
• The first cells attracted to sites of inflammation are neutrophils. • Cytokines activate vascular endothelial cells so that neutrophils in the bloodstream will stop, attach, and then migrate toward sites of microbial invasion and tissue damage. • Neutrophils bind, phagocytose, and kill invading microorganisms. • Microorganisms must usually be opsonized before they can be efficiently ingested and killed. The most effective opsonins are antibodies and complement. • Ingested microorganisms are killed by potent oxidants through a process called the respiratory burst, by antibacterial proteins called defensins and by lytic enzymes. • Neutrophils are short-lived cells that cannot undertake prolonged or multiple phagocytosis. The defensive cells of the body circulate in the bloodstream, where they are collectively called leukocytes (white cells). These leukocytes are derived from pluripotent stem cells located in the bone marrow (Figure 4-1). All types of leukocytes, including neutrophils, monocytes, lymphocytes, and dendritic cells, originate from bone marrow (myeloid) stem cells, and all help defend the body. Two types of leukocytes specialize in killing and eating invading microorganisms. These cells, called neutrophils and macrophages, originate from a common stem cell but look very different and have different, but complementary, roles. Thus neutrophils respond and eat invading organisms very rapidly but are incapable of sustained phagocytic effort. Macrophages, in contrast, move more slowly but are highly effective phagocytes and are capable of repeated phagocytosis. In this chapter, we review the properties of neutrophils and their role in inflammation and innate immunity. We will examine macrophages in the next chapter. Examination of a stained blood smear reveals many different types of leukocyte. Those that have a cytoplasm filled with granules are called granulocytes (Figure 4-2). Granulocytes also have a characteristic lobulated, irregular nucleus, so that they are described as “polymorphonuclear” (as opposed to the single rounded nucleus of “mononuclear” cells such as macrophages). Granulocytes are classified based on the staining properties of their granules. Cells whose granules take up basic dyes such as hematoxylin are called basophils; those whose granules take up acidic dyes such as eosin are called eosinophils; and those that take up neither basic nor acidic dyes are called neutrophils. All three populations play important roles in the defense of the body. The predominant blood leukocyte is the polymorphonuclear neutrophil granulocyte, otherwise called the neutrophil (Figure 4-3). About two thirds of the hematopoietic activity of the bone marrow is devoted to neutrophil production. Neutrophils are formed by bone marrow stem cells at a rate of about 8 million per minute in normal humans; they migrate to the bloodstream and about 12 hours later move into the tissues. They live for only a few days and must therefore be constantly replaced. Neutrophils constitute about 60% to 75% of the blood leukocytes in most carnivores, about 50% in the horse and 20% to 30% in cattle, sheep, and laboratory rodents. Normally, however, circulating neutrophils account for only 1% to 2% of the total population. The vast majority are sequestered in capillaries within the liver, spleen, lungs, and bone marrow. During bacterial infections, the numbers of circulating neutrophils may increase 10-fold as the stored cells are released from the bone marrow and other organs. The production of neutrophils is regulated by a cytokine called granulocyte colony-stimulating factor (G-CSF) and their loss by their rate of apoptosis. Normal neutrophils migrate to tissues, where they eventually become apoptotic and are then phagocytosed by macrophages. The production of G-CSF is regulated by their rate of apoptosis. Thus dying neutrophils are removed by macrophages. These macrophages produce interleukin-23 (IL-23) so that, as neutrophils die, IL-23 production increases. IL-23 promotes IL-17 production by lymphocytes (Th17 cells; Chapter 20). IL-17 stimulates G-CSF production and stem cell activity. As a result, the rate of neutrophil production matches the rate of their removal by apoptosis. Toll-like receptors (TLRs) are also expressed on myeloid stem cells. During microbial infections, pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharides bind to these TLRs and trigger the stem cells to produce more neutrophils. TLRs thus provide a mechanism whereby neutrophil availability increases rapidly in response to infection. Neutrophils suspended in blood are round cells about 10 to 20 µm in diameter. They have a finely granular cytosol at the center of which is an irregular sausage-like or segmented nucleus (Figure 4-4). The chromatin within the nucleus is condensed and compacted so that neutrophils do not divide. Electron microscopy shows three major types of enzyme-rich granules in the cytosol (Figure 4-5). Primary (azurophil) granules contain enzymes such as myeloperoxidase, lysozyme, elastase, β-glucuronidase, and cathepsin B. Secondary (specific) granules lack myeloperoxidase but contain lysozyme and collagenase and the iron-binding protein lactoferrin. Tertiary granules contain gelatinase. These granules are synthesized at different stages in the cell’s development. Thus primary granules are synthesized at the promyelocyte stage, secondary granules at the myeloid stage, and tertiary granules late in the development process. Granules formed early in development are rarely exocytosed, whereas tertiary granules are readily exocytosed. Because neutrophil granules contain a complex mixture of bactericidal molecules, the cells may regulate their release at inflammatory sites to ensure that they are appropriate. Neutrophil granule proteins enhance monocyte adherence to vascular endothelium, trigger macrophages to secrete cytokines, and activate dendritic cells, thus promoting antigen presentation. Neutrophil secretory vesicles and granule membranes also serve as storage sites for receptors and other membrane-integrated proteins. Mature neutrophils have a small Golgi apparatus, some mitochondria, a few ribosomes, and a little rough endoplasmic reticulum. In aggregate, the endothelial cells that line all blood vessels collectively have a huge surface area (estimated at 4000 square meters in humans) and thus serve as a broad sensor of microbial invasion. When bacterial products such as LPS or damage-associated molecular patterns (DAMPs) from damaged tissues such as thrombin or histamine reach the capillary endothelium, they stimulate the cells to express a glycoprotein called P-selectin (CD62P) on their surface. P-selectin is normally stored in cytoplasmic granules but can move to the cell surface within minutes after cell stimulation. Once expressed, the P-selectin can bind a protein called L-selectin (CD62L) on the surface of passing neutrophils. At first, this binding is transient because the neutrophils readily shed their L-selectin. Nevertheless, the neutrophils gradually slow, roll along the endothelial cell surface as they lose speed, and eventually come to a complete stop (Figure 4-6). This mainly happens in venules where the vessel wall is thin and the diameter sufficiently small to permit the neutrophils to make firm contact with the endothelium. As neutrophils roll along the endothelial surface, the second stage of adhesion occurs. Platelet-activating factor (PAF), secreted by the endothelial cells, triggers the rolling neutrophils to express an adhesive protein called CD11a/CD18 or leukocyte function–associated antigen-1 (LFA-1). LFA-1 is an integrin that binds to an intercellular adhesion molecule-1 (ICAM-1 or CD54) on the endothelial cells (Figure 4-7). This strong binding brings the neutrophil to a complete stop and attaches it firmly to the vessel wall despite the shearing force of the blood flow. Adherent neutrophils also secrete small amounts of elastase. The elastase removes CD43 (leukosialin), an antiadhesive protein, from the neutrophil surface, permitting the cells to bind even more strongly. Many cell surface proteins make cells stick together, but the most important of these are the integrins. There are several families of integrins. Each consists of paired protein chains (heterodimers) using a unique α chain linked to a common β chain. For example, three β2-integrins are found on neutrophils. Their α chain, called CD11a, b, or c, is linked to a common β2 chain (CD18). Therefore, these three integrins are called CD11a/CD18, CD11b/CD18, and CD11c/CD18. As described previously, LFA-1 on activated neutrophils binds to ICAM-1 on capillary endothelial cells. CD11b/CD18 also binds leukocytes to endothelial cells and is a receptor for some components of the complement system (complement receptor 3, CR3) (Chapter 7).
Innate Immunity
Neutrophils and Phagocytosis
Leukocyte Classification
Neutrophils
Structure
Emigration from the Bloodstream
Changes in Endothelial Cells
Changes in Neutrophils
Integrins
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree