Chapter 3 Infrared Thermography in Zoo and Wild Animals
Infrared (IR) thermography is a noninvasive diagnostic screening tool that does not require handling or restraint of an animal. Physiologic or pathologic processes involving changes in surface temperature may be evaluated using this technique. This modern method provides real-time, instantaneous visual images with measurements of surface temperatures over a greater distance.
The first medical application of “thermography” was by Hippocrates (ca. 460-375 bc), who used thin layers of mud for his temperature measurements, similar to modern thermography. An area of great heat emission caused an area of the mud to dry first, and thus a “hot spot” was detected.29 It was not until the mid–eighteenth century, however, that temperature scales were developed by Fahrenheit, Réaumur, and Celsius, and not until 1800 that Sir William Herschel discovered infrared rays distinguishable from visible light. The first detector was constructed in 1830.6
Infrared thermography has been used for skin temperature measurement in human medicine since 1960 and for the early detection of diseases since 1980, mainly pathologic processes such as pain in the lumbosacral region, intervertebral disc prolapse, spinal cord lesion, traumatic lesions, fractures, neuropathology, cardiovascular diseases (especially impairment of blood supply), lateral effects of heat or frost burns, and long-term monitoring of skin transplants. In wildlife biology, IR thermography has been used since the mid-1940s for detecting and monitoring mammal and bird species. To some degree the method could even be used successfully in animal censuses. In veterinary medicine this technique has been used on farm and companion animals since the late 1950s.9 The most advanced field is that of equine medicine.24,28,29 Eulenberger and Kämpfer3 first recommended the use of IR thermography in zoo and wild animal medicine.
Phillips15 performed the first large-scale comparative studies on thermoregulation in zoo animals with the aid of infrared thermography. Both studies employed traditional, carbon dioxide (CO2)–cooled systems, which proved to be difficult to use under routine zoo and wildlife conditions. Hilsberg9 first used IR thermography extensively with modern equipment in zoo medicine.
METHOD
Infrared thermography makes use of the physical characteristic of bodies or materials to emit electromagnetic waves, and with the aid of a special detector, these rays are visible. Therefore, surface temperatures are measured over a greater distance.6
As with other techniques, however, IR thermography presents specific challenges in zoo and wildlife medicine that are not encountered as often in human medicine and classic veterinary medicine. For example, detailed knowledge of the morphology of many different species is required; no control exists over the animal under investigation (e.g., movement, position relative to the sun, muddy or wet surface parts, positioning of animal for best investigation); and no specific examination room in a veterinary clinic with controlled environmental parameters (e.g., temperature) is available.
TECHNIQUE
The IR camera works similar to a digital video camera, except the lenses possess specific attributes. Because glass hinders the transmission of heat waves, other materials are used as semiconductors, such as germanium-zinc, lead-selenium, or cadmium-mercury-telluride. Each specific mixture of half-metals measures a defined wavelength within the IR spectrum. Each of these wavelength windows possesses specific properties, but also disadvantages, so the industry has tried to optimize the materials used for the required purposes. Gaussorgues6 provides detailed information on the physics behind these systems, with a shorter, more veterinary-oriented version by Hilsberg.9 Before obtaining a system, the clinician must consider the lens specification.
An IR system should be certified by the regional authorities. Only such systems guarantee that the measured temperatures are accurate and that it is legal to use the system; specific regulations exist because of the military use of this technology. Recently, increasing numbers of systems are appearing on the market that are remakes or copies of earlier units. These systems, however, may not be certified and thus may yield false temperature readings. The potential thermographer should consult with engineers or local experts before acquiring such equipment. All the studies described by Hilsberg9 have used the IR systems by the companies AGEMA and later FLIR Systems. With the enormous technical developments achieved in the last decade, this technique should be used throughout veterinary medicine, especially in zoo and wild animal medicine, as an aid in primary diagnostics.
SOURCES OF ARTIFACTS
Clipped hair may increase temperature readings. Alcoholic ointments or other surface heat–producing materials also create artifacts in the form of increased heat emission. On the other hand, cold water, dirt, or mud may create an altered heat emission that shows lower temperatures, at least when first applied. Later, this foreign material emits the heat according to its composition. Additionally, uneven pelage creates uneven heat transmission. Strong physical activity of the animal will create local heat production at first, but heat emission from the whole-animal surface may occur later, depending on the type of animal and the type and duration of the activity.
High ambient temperature poses difficulties when looking for smaller temperature differences. Under high ambient temperatures the difference between the animal core and surface temperature decreases. This makes the use of IR thermography more challenging in field investigations than in zoo settings. A good way to address this problem is using the technique in a stable or, for wildlife at night, near a waterhole. The sun itself also creates significant artifacts, and therefore cloudy days are preferred. However, clouds still allow a certain quantity of infrared emission. The effect of the sun is especially visible in giraffes and zebras. In zebras the author found specific skin pattern–related heat radiation when the animals were in their stables at night.1
GENERAL FIELDS OF USE
Thermoregulation: the Basics for Medical Thermography
The only exceptions from this uniform surface temperature are the obligatory or facultative thermal windows. In mammals the eyes are always obligatory thermal windows, as are the mouth, heart region, and the rectal and vaginal openings, as well as the penis during urination or erection. These are areas where function permits no insulation, or where an opening in the body is connected with the body core. Facultative thermal windows are much more difficult to judge because they may or may not be active, depending on the ambient conditions and the thermoregulatory needs of each animal. These are species specific and may also show individual variations.
Therefore, it is advisable to study many individuals over time before judging pathologic processes. When this is not possible, the investigator should make use of other individuals of the same species in the same environment, or if time permits, investigate the same individual on different occasions under similar conditions. This last approach yields the most accurate investigation technique for an individual. This is the technique used in equine preventive medicine or in racecourse training management, especially in Great Britain. Experienced trainers and veterinarians are able to identify potentially lame animals up to 2 weeks before the animal actually shows clinical signs.13,22,27
General indicators of altered thermoregulation can be physiologic or pathologic, as follows:
Elephants
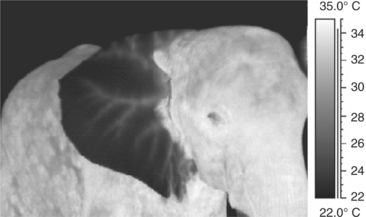
As animals without a notable amount of hair on the body, elephants display relatively even heat radiation over their entire body surface when in a thermoneutral zone. Only the ears show less heat radiation than the body, whereas the eyes, mouth, and anus are thermal windows (Figure 3-1). Any other source of heat should be investigated. A thermogram of an elephant feeding on branches may show the “hot” mouth, the hot distal trunk, and the warm tips of the front feet. Thermograms of an African (Loxondonta africana) and an Asian (Elephas maximus) elephant may show heat radiation with specific reference to their ears. In both animals the larger blood vessels are localized.
Intense sunshine creates high temperature readings on the body and outer surface of the ears, especially in African elephants. The underside of the large ears usually remains cool. Ear flapping results in increased convection and saves the ears from collecting further heat. More than 30% of excess heat may be radiated off the ears in African elephants.16 In a group of Asian elephants in a newly built indoor enclosure, the keepers noted that the elephants did not display normal activity patterns but seemed somewhat lethargic. IR thermography revealed an altered thermoregulation, with ears that were the same high temperature as the body. This was noted in all members of the group. The ambient high humidity of 95% was reduced, and the animals were given more frequent access to cool water. Overheating poses a great stress and health risk to captive elephants, especially Asian elephants, and may even cause death during immobilization, if the thermoregulatory influence of a new enclosure is not evaluated; in this case the health of the animals improved.9 Uhlemann25,26 provides similar examples of recent investigations into thermoregulatory behavior of zoo animals involving insight into environmental heat stress caused by enclosure design.
Elephants may also display increased radiation from parts or whole ears caused by psychologic stress. When this occurs, at least some animals in the herd display normal ear radiation and serve as comparisons.9
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree