Chapter 17 Amphibian Chytridiomycosis
Chytridiomycosis is an important emerging disease of amphibians caused by the chytrid fungus Batrachochytrium dendrobatidis (Bd). Although previously classified within the kingdom Protista, organisms in the phylum Chytridiomycota (“chytrids”) are now known to be true fungi that produce characteristic, motile, posteriorly uniflagellate zoospores within discrete fungal bodies termed thalli. Chytrids are ubiquitous in aquatic environments, and although several species are known pathogens of plants, fungi, or invertebrates, none were reported to infect vertebrates until the description of Bd in amphibians.2,21 To date, only Bd has been associated with amphibian disease, and Koch’s postulates have been fulfilled.27
Preliminary reports of chytridiomycosis in the late 1990s described Bd as either an aquatic, fungal-like protist or a Perkinsus-like protozoan. In addition, some reports of infection with Basidiobolus ranarum in toads were subsequently determined to have been caused instead by infection with Bd.24,31 Infection has now been recognized in a wide range of both anuran (frogs and toads) and urodele (salamanders and newts) amphibians. A report of infection in the order Gymniophona (caecilians) is anecdotal and needs to be confirmed.25
Although a significant disease problem in both captive and free-ranging animals, chytridiomycosis is most important because of an association with mass mortality events and population declines in the United States, Europe, Latin America, and Australia.2,5,7,12,24 Concerns about the effects of chytridiomycosis on free-ranging populations, including the possibility of disease-associated extinctions, have resulted in calls for ex situ species salvage and breeding programs that may significantly impact zoologic collections.
CHYTRIDIOMYCOSIS AND AMPHIBIAN POPULATION DECLINES
Amphibian population declines have been increasingly recognized since the late 1980s and have generated much public and scientific interest. A recent survey suggests that 32.5% of known amphibian species are globally threatened and 43.2% are experiencing a population decrease.38 Although in some declines a clear anthropogenic cause such as habitat loss or species exploitation may be implicated, in many others the cause is not obvious (“enigmatic decline”). Of the enigmatic-type population declines, chytridiomycosis and climate change are most frequently cited as potential causes.
Population declines attributed to chytridiomycosis are best documented in stream-dwelling species at high elevations in the rain forests of Central America and eastern Australia.19,41 In both locations, evidence suggests temporal and geographic progression of declines.8 The apparent southward progression of disease incidence in Costa Rica and Panama has allowed for prediction of future sites of decline.20 Disease progression may have significant implications for worldwide amphibian species diversity because up to half of all species live in neotropical regions that environmental modeling suggests could be a suitable niche for Bd.37
It is unclear whether Bd is a novel pathogen that has recently been introduced to naive populations or an endemic pathogen that has emerged because of environmental or other cofactors.35 It may be that both circumstances exist, depending on geographic location. Preliminary genetic information obtained from isolates originating from several continents indicates that Bd is a recently emerged clone, possibly consistent with an introduced novel pathogen.23 Research on the means of introduction of Bd to new locations has focused on international movement of amphibians for food, laboratory research, and the pet trade. In particular, the African clawed frog (Xenopus laevis) and the bullfrog (Rana catesbeiana) are species that have been widely moved or introduced worldwide and are good potential reservoir hosts for Bd because they carry infection without significant clinical signs.9,40 The occurrence of Bd-associated mortality events at lower temperatures (within preferred temperature ranges for Bd) suggests that environmental cofactors may play a role in mortality events resulting from either recent introduction of Bd to a region or exacerbation of endemic infections.3,33 Stable endemic Bd infection of populations has been documented after catastrophic declines presumed to have resulted from introduced Bd infection,36 as well as in populations without documented declines.28
PATHOLOGY AND PATHOGENESIS
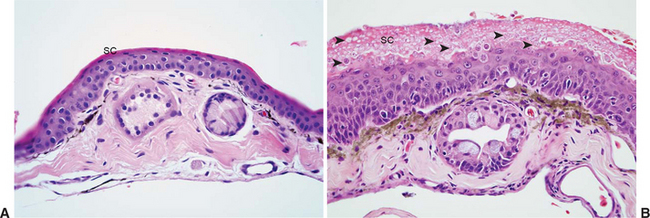
Lesions of chytridiomycosis are limited to keratinizing epithelium in the skin of postmetamorphic animals and the mouthparts (tooth rows and jaw sheaths) of tadpoles.2,32,34 Dissemination to deeper portions of the skin or to viscera does not occur. Lesions consist of varying degrees of epidermal hyperplasia and hyperkeratosis, with intralesional thalli characteristic of Bd (Figure 17-1). The keratinized layers (stratum corneum) of amphibian skin are usually very thin, and hyperkeratosis may be overlooked if using criteria established for other species. Associated inflammatory cell infiltrates are an inconsistent finding and, when observed, are usually in association with severe or chronic infections or in cases with secondary bacterial or fungal infection. Secondary infections are common, presumably because environmental bacteria and fungi become trapped in excessive keratin layers or within empty thalli of Bd that have expelled zoospores. The severity and distribution of lesions may range from relatively minimal focal lesions in subclinically infected animals13 to severe, multifocally extensive to diffuse lesions considered to be clinically significant.
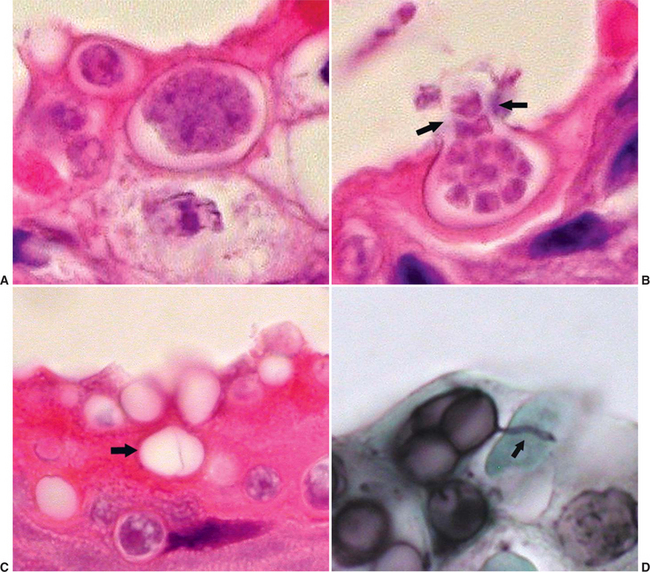
Characteristic features of Bd thalli aid in identification in cytologic preparations or histologic section. The spherical thalli, which are intracellular in superficial keratinocytes, range from approximately 7 to 20 μm in diameter, and mature thalli (zoosporangia) may contain discrete, 1- to 2-μm basophilic zoospores. Empty thalli that have discharged their zoospores are common and should be distinguished from cross sections of fungal hyphae or ducts of cutaneous glands. Flask-shaped thalli with prominent discharge papillae (discharge tubes) can usually be found in most heavy infections (Figure 17-2). Colonial thalli have evidence of fine internal septation and are best appreciated in empty thalli or in Gomori’s methenamine silver (GMS)–stained sections. An inconsistent, but sometimes helpful, finding in GMS–stained sections are thin, rootlike extensions from thalli termed rhizoids (Figure 17-2, D).
The mechanism by which Bd causes death of susceptible animals is still unclear. Disruption of normal skin function, especially in regard to water absorption and electrolyte balance, and secretion of a mycotoxin are the most frequently cited hypotheses. Some clinically affected animals show evidence of dehydration and hemoconcentration, which may provide support for disruption of skin function.11 Death in Bufo boreas tadpoles shortly after exposure to cultures of Bd might support the role of a mycotoxin.4 There is evidence for both species-related and age-related differences in susceptibility to clinically significant infections.10,13,18
Transmission of Bd infection is through the motile, flagellated zoospores and may occur by direct contact between animals or by contact with water or substrates used in housing affected animals. Extension of infection from the keratinized mouthparts of tadpoles may occur as the tadpoles metamorphose and develop a keratinized epidermis.18,34
CLINICAL SIGNS
Clinical signs of chytridiomycosis are variable and range from unexpected death without premonitory signs to animals with evidence of significant skin disease. The most common cutaneous signs of chytridiomycosis are excessive shedding (“sloughing”) of skin, rough or granular changes in skin texture, and brown to red (hyperemia) skin discoloration. Findings are most often distributed on the ventral body and feet of terrestrial animals, but may be diffuse in totally aquatic species. Hyperemia and other features, such as cutaneous ulceration, are more common in animals with secondary bacterial, fungal, or water mold (Oomycetes) infections. In these cases, chytridiomycosis should be distinguished from other potential causes of “red leg” syndrome, including bacterial septicemia and iridovirus infection.
Tadpoles infected with Bd are usually asymptomatic and may serve as reservoirs of infection for postmetamorphic animals. Detailed gross examination of the keratinized mouthparts may show areas of depigmentation, which should be distinguished from other potential causes of mouthpart abnormality, including chemical exposure and low environmental temperature.34 Reduced body mass of infected tadpoles and deaths of some individuals in acute-exposure studies have been reported for selected species.4,30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree