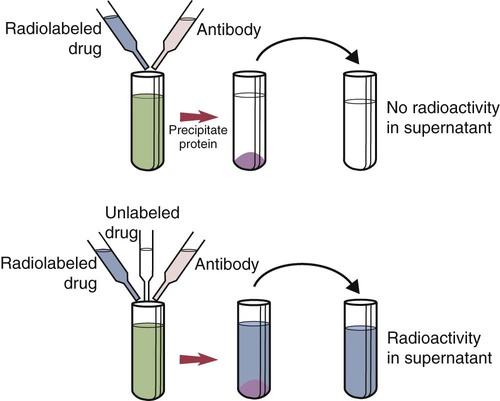
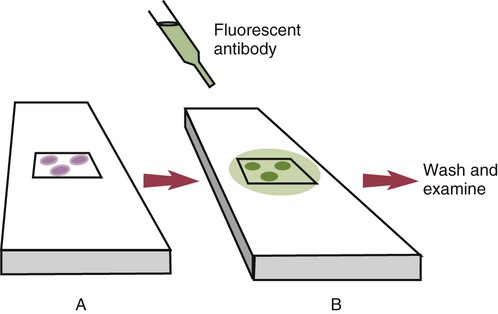
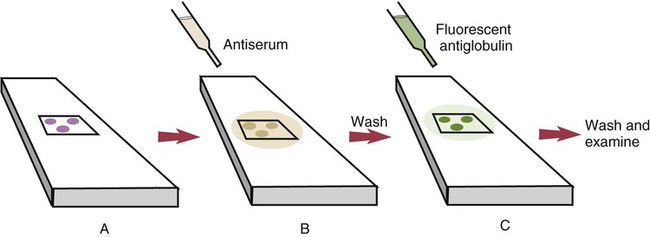
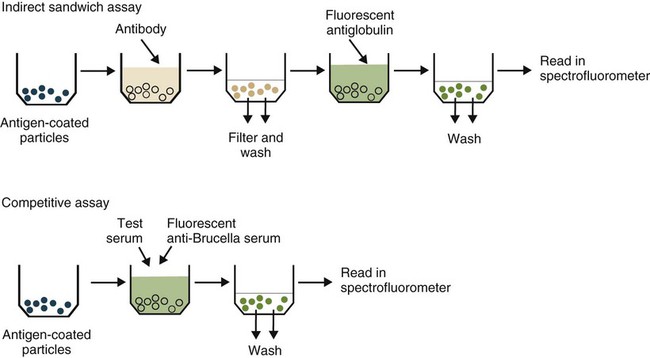
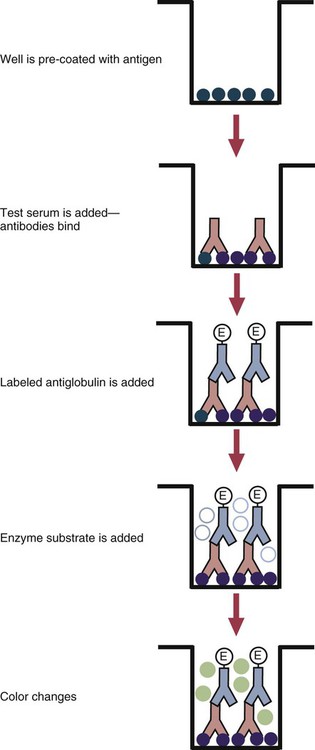
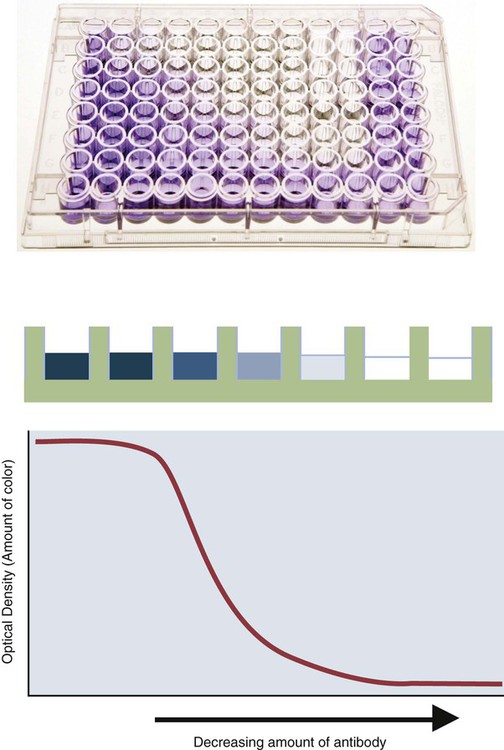
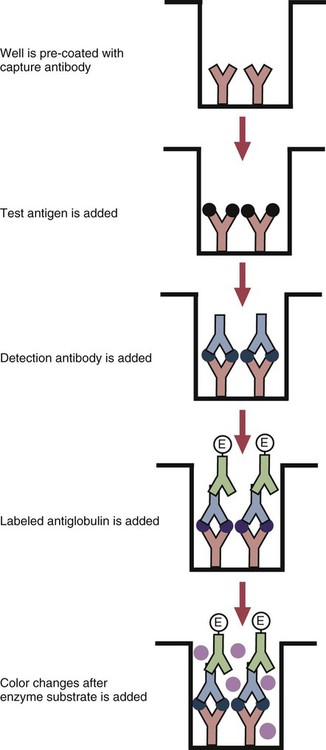
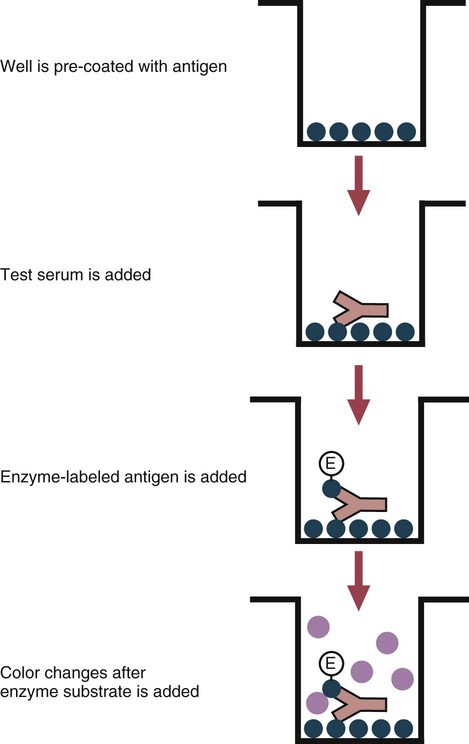
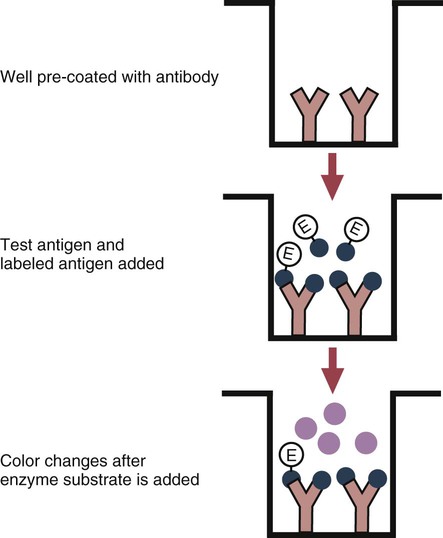
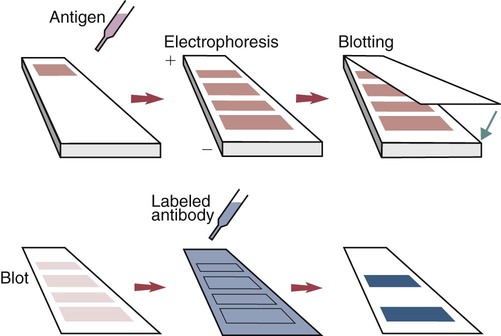
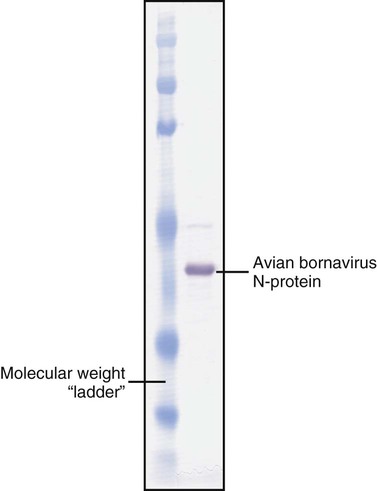
• The detection of antibodies in serum may be of assistance in the diagnosis of an infectious disease. Specific antibodies may also be used to identify the presence of an antigen. • The most sensitive and specific tests are those that directly detect the antigen or antibody of interest. These are called primary binding tests. An example of a primary binding test is an enzyme-linked immunosorbent assay (ELISA). • Secondary binding tests tend to be the easiest to perform but are less sensitive than primary binding tests. Examples include precipitation and agglutination tests. • Tertiary tests directly measure protection. They are usually complex and so may not lend themselves to rapid testing. An example is a virus neutralization test. • Serological tests are judged by the number of false-positive results they generate (their specificity) and by the number of false-negative test results they generate (their sensitivity). • In general highly sensitive tests tend to have low specificity, and vice versa. Serological techniques can be classified into three broad categories. Primary binding tests directly measure the binding of antigen to antibody (Table 41-1). Secondary binding tests measure the results of antigen-antibody interaction in vitro. These tests are usually less sensitive than the primary binding tests but may be simpler to perform or require less complex technology. The third category, in vivo tests, measure the actual protective effect of antibodies in an animal. Table 41-1 Smallest Amount of Antibody Protein Detectable by Selected Immunological Tests Hybridoma-derived monoclonal antibodies are pure and specific, can be used as standard chemical reagents, and can be obtained in almost unlimited amounts (Chapter 15). As a result, monoclonal antibodies frequently replace conventional antiserum as reagents in immunodiagnostic tests. Competitive immunoassays are based on the principle that unlabeled antigen will displace radiolabeled antigen from immune complexes (Figure 41-1). These tests are exquisitely sensitive and are commonly used to detect trace amounts of drugs. The antigen (or drug) is labeled with a radioactive isotope such as tritium (H3), carbon-14, or iodine-125. When radiolabeled antigen is mixed with its specific antibody, the two combine to form immune complexes that can be precipitated out of solution. Any radioactivity remaining in the supernatant fluid is due to the presence of unbound antigen. If unlabeled antigen is added to the mixture, before adding the antibody, it will compete with the radioactive antigen for antibody-binding sites. As a result, some labeled antigen will be unable to bind, and the amount of radioactivity in the supernatant will increase. If a standard curve is first constructed based on the use of known amounts of unlabeled antigen, the amount of antigen in a test sample may be measured by reference to this standard curve. Direct fluorescent antibody tests are used to identify the presence of antigen in a tissue sample. Antibody directed against a specific antigen such as a bacterium or virus is first labeled with FITC. A tissue section or smear containing the organism is fixed to a glass slide, incubated with the labeled antiserum, and then washed to remove any unbound antibody (Figure 41-2). When examined by darkfield illumination under a microscope with an ultraviolet light source, the organisms that bind the labeled antibody will fluoresce brightly. This test can identify the presence of small numbers of bacteria in a sample. For example, it can be used to detect M. avium subspecies paratuberculosis in feces, or to detect bacteria such as Dichelobacter nodosus, Listeria monocytogenes, or clostridia in diseased tissues (Figure 41-3). It may also be employed to detect viruses in tissue culture or in tissues from infected animals. Examples include the detection of rabies virus in the brains of infected animals or feline leukemia virus in infected cat leukocytes (Figure 38-3). Indirect fluorescent antibody tests can be used to measure antibodies in serum or to identify specific antigens in tissues or cell cultures. When measuring antibody levels, antigen is employed as a tissue smear, section, or cell culture on a slide or coverslip. This is incubated in serum suspected of containing antibodies to that antigen. The serum is then washed off, leaving only specific antibodies bound to the antigen (Figure 41-4). These bound antibodies may then be visualized by incubating the smear in FITC-labeled antiglobulin. When the unbound labeled antiglobulin is removed by washing and the slide examined, the presence of fluorescence indicates that antibody was present in the test serum. The quantity of antibody in the test serum may be estimated by examining increasing dilutions of serum on different antigen preparations. Immunofluorescence assays can be automated and quantitated by means of particle immunoassays (Figure 41-5). For example, antigen-coated, submicrometer polystyrene particles can be mixed with test serum. After incubation, the particles are recovered by vacuum filtration, washed to remove unbound antibody, and exposed to a fluorescent antiglobulin. After filtering the suspension again and washing to remove unbound antiglobulin, the particle suspension can be placed in a spectrofluorometer and the intensity of particle-bound fluorescence measured. This provides a measure of the level of antibodies in the test serum. A very useful variation on this is the competitive assay used as a rapid test for antibodies to Brucella abortus in cattle. In this case, Brucella antigen-coated polystyrene particles are mixed with a standard amount of fluorescent anti-Brucella serum and the serum under test. If positive, the unlabeled test serum blocks the binding of fluorescent antibodies to the particles. The more antibody in the test serum, the greater is the inhibition of fluorescent antibody binding. The most common form of ELISA is used to detect and measure specific antibodies. In order to perform this assay, microwells in polystyrene plates are first filled with an antigen solution (Figure 41-6). Proteins bind firmly to polystyrene surfaces, so that after unbound antigen is removed by vigorous washing, the wells remain coated with a layer of antigen. These coated plates can be stored until required. The serum under test is added to the wells. Any antibodies in the serum will bind to the antigen layer. After incubation and washing to remove unbound antibody, the presence of any bound antibodies can be detected by adding a solution containing an antiglobulin chemically linked to an enzyme. This labeled antiglobulin binds to the antibody and, following incubation and washing, can be detected and measured by adding a solution containing the enzyme substrate. The enzyme and substrate have been selected to ensure that a colored product develops in the tube. The intensity of the color that develops is therefore proportional to the amount of enzyme-linked antiglobulin that is bound, which in turn is proportional to the amount of antibody present in the serum under test. The color intensity may be estimated visually or, preferably, by spectrophotometry. One modification of this technique is the antibody sandwich ELISA, which can be used to detect and measure a specific antigen (Figure 41-7). The wells in polystyrene plates are coated with specific antibody (capture antibody) before testing. To conduct the test, the antigen solution to be tested is added to each well. The capture antibody will bind any antigen present in the test solution. This step is followed, after washing, by specific antibody, which also binds the antigen (the detection antibody). After washing to remove unbound antibody, enzyme-labeled antiglobulin, and substrate, as described for the indirect technique, are added. (It is important that the capture antibody and the detection antibody are from a different species and that a species-specific antiglobulin is used for visualization of the detection antibody. This will avoid false-positive results caused by binding of the antiglobulin to the capture antibody in the absence of antigen.) In this assay, the intensity of the color reaction is related directly to the amount of bound antigen. Because these tests involve the formation of antibody-antigen-antibody layers, they are called sandwich ELISAs. Sandwich ELISAs are used to detect circulating virus in blood from cats with feline leukemia. Another common modification of this technique is the labeled-antigen ELISA used to detect antibodies. This is favored in manufactured diagnostic kits. The antigen is bound to the microwells before testing (Figure 41-8). The serum to be tested is added, followed, after washing, by labeled antigen. Any serum antibodies present will bind the labeled antigen to the microwell where it can be measured. A competitive ELISA can be used to measure hapten molecules or viral antigens (Figure 41-9). In this technique, the microwell is coated with specific antibody before testing. In a single reaction, the test sample and enzyme-labeled antigen are placed in the well where the antigens compete for the antibody-binding sites. The amount of labeled antigen bound to the microwell is inversely related to the concentration of antigen in the test sample. This technique is faster than other ELISA techniques. It can be made very sensitive if the sample antigen is permitted to react with the antibody before the labeled antigen is added. One solution to the problem of identifying protein antigens in a complex mixture is by use of a technique called Western blotting. This is a three-stage primary binding test (Figure 41-10). Stage 1 involves electrophoresis of a protein mixture on gels so that each component is resolved into a single band. Stage 2 involves blotting or transfer of these protein bands to an immobilizing nitrocellulose membrane. This is accomplished by placing the membrane on top of the gel and sandwiching the two between sponges saturated with buffer. The membrane-gel sandwich is supported between rigid plastic sheets and placed in a buffer reservoir, and an electrical current is passed between the sponges. The protein bands are transferred from the gel to the membrane without loss of resolution. The third stage involves visualization of transferred antigens by means of an enzyme immunoassay or radioimmunoassay. When an enzyme immunoassay is employed, the membrane is first incubated in specific antiserum. After the membrane has been washed, an enzyme-labeled antiglobulin solution is added. When this is removed by washing, substrate is added, and a color develops in the bands where the antibody has bound to antigen. When isotope-labeled antiglobulin is used, an autoradiograph must be made and the labeled band identified by darkening of a photographic emulsion. Western blotting is used to identify the important antigens in complex microorganisms or parasites (Figure 41-11). A variant form of the Western blot is the dot blot. Antigen solution is drawn through a nitrocellulose membrane so that any protein binds to the membrane. The presence of the antigen can be determined using specific antiserum and enzyme-labeled antiglobulin in sequence. After exposure to enzyme substrate, the presence of a stained dot is a positive reaction. (Use of nasal washings as a source of the antigen, such as when trying to detect respiratory viruses, is called a snot-blot!).
Immunodiagnostic Techniques
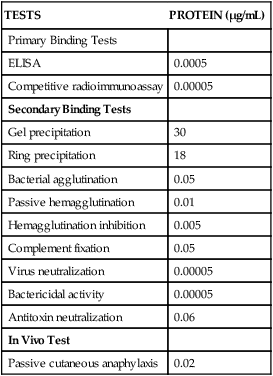
TESTS
PROTEIN (µg/mL)
Primary Binding Tests
ELISA
0.0005
Competitive radioimmunoassay
0.00005
Secondary Binding Tests
Gel precipitation
30
Ring precipitation
18
Bacterial agglutination
0.05
Passive hemagglutination
0.01
Hemagglutination inhibition
0.005
Complement fixation
0.05
Virus neutralization
0.00005
Bactericidal activity
0.00005
Antitoxin neutralization
0.06
In Vivo Test
Passive cutaneous anaphylaxis
0.02
Reagents Used in Serological Tests
Serum
Monoclonal Antibodies
Radioimmunoassays
Radioimmunoassays for Antigen
Immunofluorescence Assays
Direct Fluorescent Antibody Tests
Indirect Fluorescent Antibody Tests
Particle Concentration Fluorescence Immunoassays
Immunoenzyme Assays
Microwell Enzyme-Linked Immunosorbent Assay Tests
Western Blotting
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunodiagnostic Techniques
Only gold members can continue reading. Log In or Register to continue