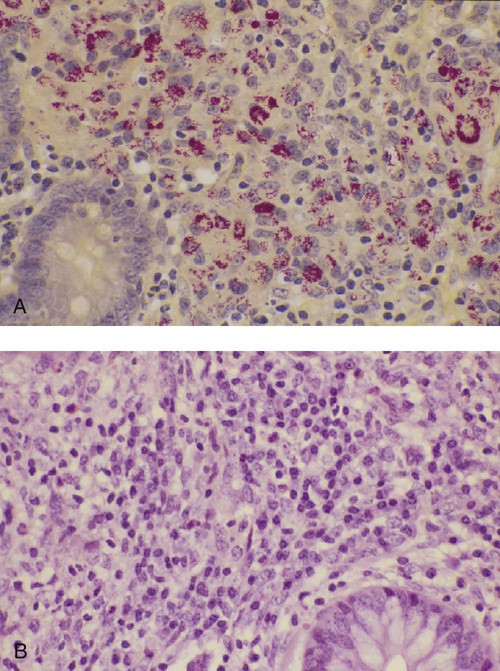
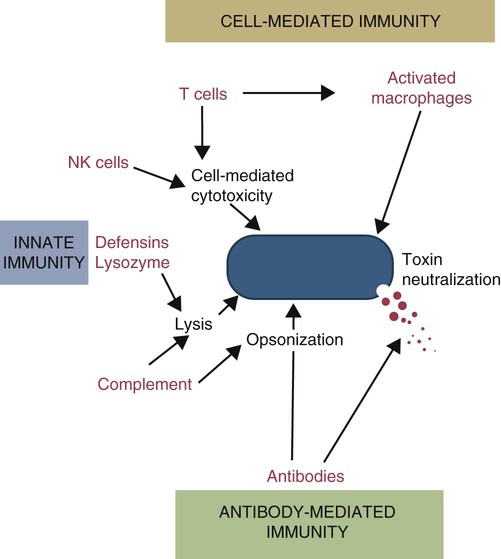
• Antibodies can neutralize bacterial toxins. • Antibodies alone will opsonize bacteria. Antibodies and complement may opsonize bacteria or kill them directly through the terminal complement complex. • T cell–mediated activation of macrophages is required to kill intracellular bacteria. • Under some circumstances, especially in mycobacterial disease, an inappropriate Th2 response rather than the required Th1 response may lead to severe disease and death. • Bacteria may resist destruction by multiple mechanisms. • Cell-mediated immune responses are usually required to protect against fungal infections. There are five basic mechanisms by which the adaptive immune responses combat bacterial infections (Figure 25-1): (1) neutralization of toxins or enzymes by antibody; (2) killing of bacteria by the classical complement pathway; (3) opsonization of bacteria by antibodies and complement, resulting in their phagocytosis and destruction; (4) destruction of intracellular bacteria by activated macrophages; and (5) direct killing of bacteria by cytotoxic T cells and NK cells. The relative importance of each of these processes depends on the species of bacteria involved and on the mechanisms by which they cause disease. As discussed in Chapter 18, some bacteria such as Brucella abortus, Mycobacterium tuberculosis, Campylobacter jejuni, R. equi, Listeria monocytogenes, Corynebacterium pseudotuberculosis, C. burnetii, and some serotypes of S. enterica can grow readily inside macrophages. In addition, L. monocytogenes can travel from cell to cell without exposure to the extracellular fluid through cytoskeletal membrane protrusions. Autophagy, as described in Chapter 4, is also a key component of the destruction of intracellular bacteria. The same cellular machinery used to destroy unwanted organelles can be employed to eliminate intracellular organisms. Autophagy (or more correctly, xenophagy) may also play a key role in delivering microbial antigens to the appropriate major histocompatibility complex (MHC) molecules. Protection against intracellular bacteria is mediated by macrophages activated through the M1 pathway. Classically activated M1 macrophages are responsive to inflammatory cytokines and microbial products (Chapter 5). Although macrophages from unimmunized animals cannot usually destroy these bacteria, this ability is acquired about 10 days after onset of infection once the macrophages are activated (Chapter 18). IFN-γ, especially in association with TNF-α, greatly enhances the production of cytokines such as TNF-α, IL-6, IL-1β and IL-12, enzymes such as indoleamine 2,3-dioxygenase (IDO) and nitric oxide synthase 2 (NOS2), and the release of reactive oxygen and nitrogen intermediates. M1 polarization has been shown to be important in resistance to L. monocytogenes, S. enterica Typhi and Typhimurium, mycobacteria, and chlamydia. For example, IFN-γ and TNF-α produced by primed T cells generate M1 macrophages, acidify their phagosomes, and kill mycobacteria. Uncontrolled M1 activation by organisms such as streptococci and E. coli, however, can contribute to pathology by inducing, for example, sepsis, tissue damage, and organ failure. The response of these activated macrophages tends to be nonspecific, particularly in listerial infections, and M1 macrophages are able to destroy many normally resistant bacteria. Thus an animal recovering from an infection with L. monocytogenes develops increased resistance to infection by M. tuberculosis. The development of M1 macrophages often coincides with the appearance of delayed (type IV) hypersensitivity responses to intradermally administered antigen (Chapter 31). The immune response clearly influences the course and severity of an infection. At best, it will result in a cure. In the absence of a cure, however, the infection may be profoundly modified. Much depends on whether a cell-mediated or antibody response is generated. Thus the type of helper T cells induced during infection may affect the course of disease. As described in Chapter 18, cell-mediated responses are required to control intracellular bacteria since only activated macrophages can prevent their growth. Macrophage activation requires that Th1 cells produce IFN-γ. Once activated, these M1 cells can localize or cure these infections. If an animal mounts an inappropriate Th2 response, cell-mediated immunity fails to develop, M2 macrophages are generated, and chronic progressive disease may result. This is readily seen in mycobacterial diseases. For example, in humans, leprosy occurs in two distinct forms called tuberculoid and lepromatous leprosy. Tuberculoid (or paucibacillary [PB]) leprosy is characterized by an intense cell-mediated immune response dominated by Th1 cells and M1 macrophages. Lesions of this form of the disease contain very few organisms. Lepromatous (or multibacillary [MB]) leprosy, in contrast, is characterized by very high antibody levels and poor cell-mediated responses. Humans with lepromatous leprosy employ Th2 cells secreting IL-4 and IL-10. The IL-10 reduces the production of IL-12, which in turn decreases IFN-γ secretion by Th1 cells and generates M2 macrophages. This reduces the patient’s ability to control Mycobacterium leprae, and their lesions contain enormous numbers of bacteria. The prognosis of lepromatous leprosy is much poorer than for tuberculoid leprosy. A similar diversity of lesions is seen in Johne’s disease of sheep. Some animals develop MB disease, in which their intestinal lesions contain enormous numbers of bacteria (Figure 25-3) and little histological evidence of a cell-mediated response. Their granulomas tend to lack organization, with large numbers of bacteria-laden macrophages intermixed with lymphocytes. In contrast, other sheep may develop PB disease, in which the lesions contain very few bacteria but large numbers of lymphocytes. These are organized nodular lesions with epithelioid cells and multinucleated giant cells at the center surrounded by fibrous connective tissue. The two forms of the disease are associated with differential expression of cytokine and chemokine receptors. Thus animals with the PB disease have increased numbers of CD25+ T cells that produce more IL-2 and much more IFN-γ than sheep with the MB form of the disease (Figure 25-4). In contrast, sheep with the MB disease have higher antibody levels and a lack of cellular immune responses. It is likely, therefore, that sheep with PB lesions mount an immune response in which Th1 cells predominate, whereas those with MB disease use Th2 cells.
Immunity to Bacteria and Fungi
Adaptive Immunity
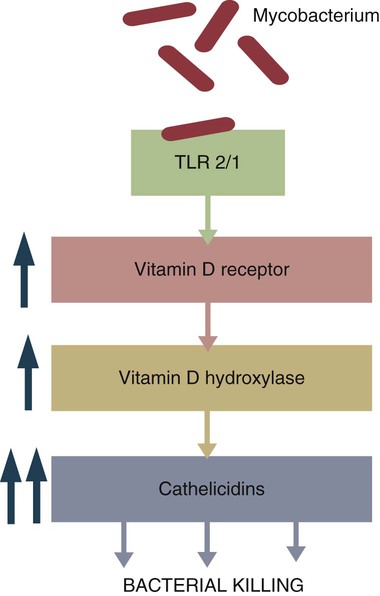
Immunity to Intracellular Bacteria
Modification of Bacterial Disease by Immune Responses
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunity to Bacteria and Fungi
Only gold members can continue reading. Log In or Register to continue