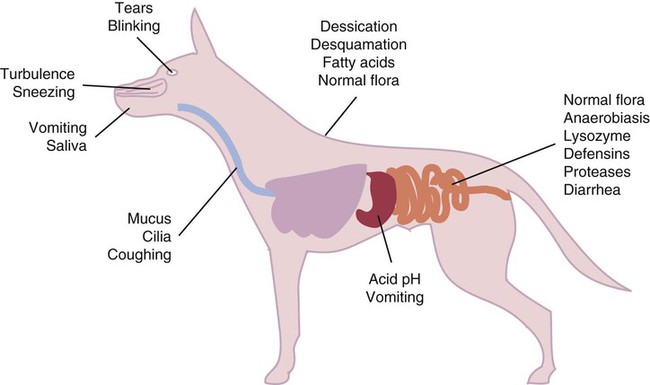
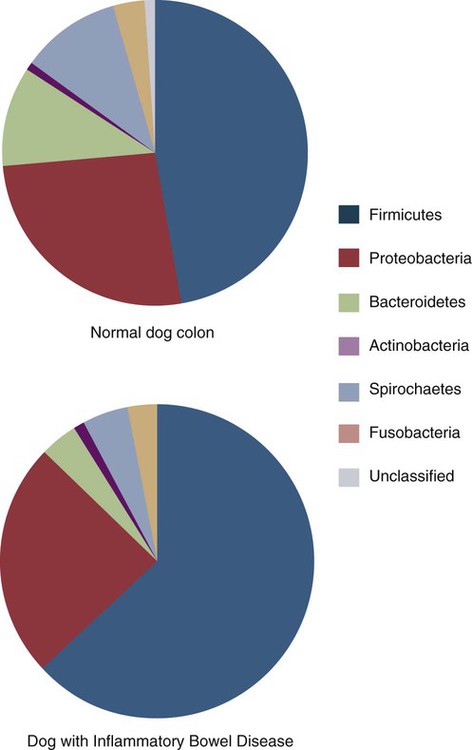
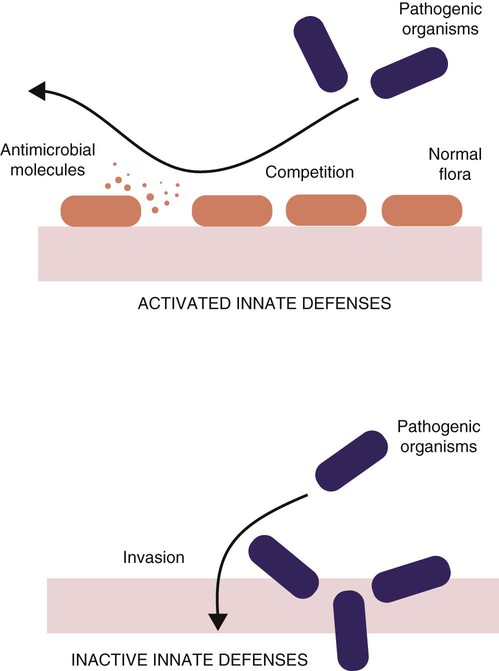
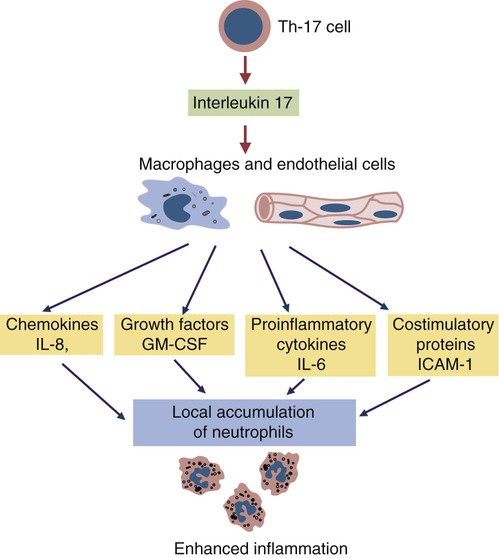
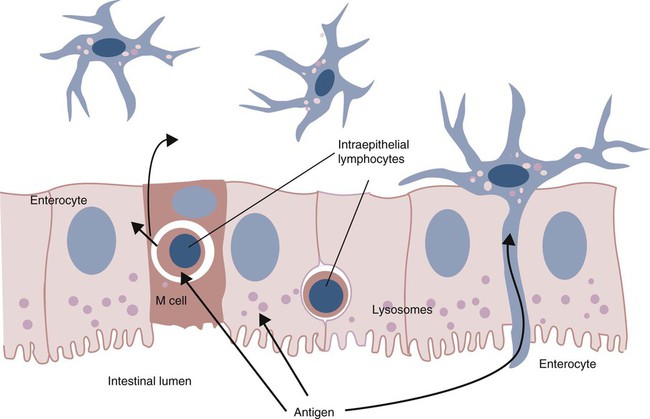
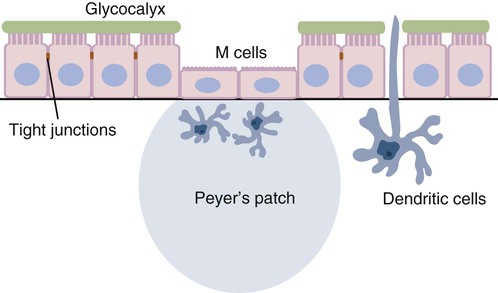
• Because it is essential to prevent microbial invasion of the body, many of its immune defenses are located on surfaces such as those in the respiratory and digestive tracts. • Surface defenses exclude invaders by preventing them from penetrating surface epithelia. • γ/δ T cells are T cells that are specialized for epithelial defense. • Immunoglobulin A (IgA), the major immunoglobulin produced on surfaces, prevents microbial invasion by blocking pathogen adherence. • IgE is a backup surface defense. It triggers local acute inflammation in response to parasites that have succeeded in evading IgA. • The normal intestine does not mount an inflammatory response to the intestinal microflora unless these organisms succeed in penetrating the intestinal wall. • The body responds in a limited fashion to antigens in food. However, unless allergies develop, this is rarely noticed. Although mammals possess an extensive array of innate and adaptive defense mechanisms within tissues, it is at their surfaces that invading microorganisms are first encountered and largely repelled or destroyed (Figure 22-1). Although the skin is the most obvious of these surfaces, it in fact represents only a small fraction of the area of the body exposed to the exterior. The areas of the mucous membranes of the intestine and respiratory tracts are at least 200 times larger. While the immune systems ensure that the interior of the body remains free of microbial invaders, it is not possible to keep the surfaces of the body sterile. Indeed a vast number of microorganisms live in a symbiotic relationship on body surfaces. These organisms are not a major threat to healthy individuals. Many are commensals whose presence is beneficial. This complex microflora is present in large numbers on all the body’s surfaces, including the skin, gastrointestinal tract, respiratory tract, and urogenital tract. The presence of large stable populations of symbiotic bacteria implies that their elimination is neither feasible nor desirable. The immune system must therefore adapt to the presence of this microflora while at the same time retaining its ability to fight pathogens. The first bacteria to colonize body surfaces are acquired by a newborn animal from its mother at the time of birth. Within days, these are followed by many different environmentally acquired bacteria, generally as a result of chance microbial encounters. These organisms compete and adapt to their environment so that over the first months of life, their composition gradually changes to the stable adult-like microflora. The size and complexity of this microflora is greatest in the gastrointestinal tract. Most intestinal microbes are bacteria (Gram-positive and Gram-negative, facultative aerobic and anaerobic), but there are some archaea, a few eukaryotes, and many viruses and bacteriophages. The composition of the microflora varies among surfaces and even among locations on those surfaces. Additionally, the composition of the intestinal microflora differs among individuals and depends on their diet. The bacterial density is relatively low in the stomach but rises in the rumen, the distal small intestine, and especially the colon (Figure 22-2). The rumen mainly contains anaerobic bacteria, with some protozoa and fungi. In the large intestine, the microflora mainly consists of strict anaerobes. It has been estimated that this microbial population can eventually reach a density of 1012 organisms per gram of intestinal contents. The first cellular barrier to microbial invasion consists of intestinal epithelial cells (enterocytes). These cells maintain barrier integrity by having tight junctions between cells and a coating of attached mucins that form a glycocalyx, and they produce multiple antimicrobial peptides. Specialized intestinal epithelial cells called Paneth cells express toll-like receptors (TLRs) (Figure 22-3). When triggered by the microflora, they secrete multiple antimicrobial peptides. These enteric defensins, known as cryptdins, accumulate within intestinal crypts and achieve very high local concentrations. They prevent commensals from entering the crypt space and so protect enterocytes from invasion. In cattle, expression of cryptdin genes occurs throughout the small intestine and colon. These cryptdins are secreted as active molecules, as opposed to the human and mouse molecules that are secreted as inactive precursors and subsequently activated by trypsin within the intestine. Parasitic or other intestinal infections may increase production of α- and β-defensins. Cryptdins serve a barrier function since they are found in highest concentrations in the fluid layer closest to the epithelium and therefore reduce microbial contact with enterocytes. The cryptdin mixture selectively kills some bacterial species and as a result regulates the precise composition of the microflora. The intestinal microflora acts competitively against potential invaders and supplements the physical defenses of this system (Figure 22-4). By occupying and exploiting the intestinal microenvironment, commensals block subsequent colonization by pathogenic bacteria. (It is, for example, possible to block Salmonella species colonization of the chicken intestine by administering an appropriate mixture of commensal bacteria to birds.) The microflora determines local environmental conditions by, for example, keeping the pH and oxygen tension low. The microflora is also influenced by the diet; as a result, the intestine of milk-fed animals is colonized largely by lactobacilli, which produce bacteriostatic lactic and butyric acids. These acids inhibit colonization by potential pathogens such as Escherichia coli, so young animals suckled naturally tend to have fewer digestive disturbances than animals weaned early in life. Genetically identical pigs housed in an outdoor environment, an indoor environment, or experimental isolators have had their intestinal flora studied. It has been found that 90% of the bacteria within the intestine of the outdoor group were Firmicutes, especially lactobacilli. In contrast, Firmicutes constituted less than 70% of the intestinal flora in the indoor group and about 50% in the isolated pigs. Pigs from cleaner environments had smaller proportions of lactobacilli. There is a strong negative correlation between the level of lactobacilli and the level of pathogens in the intestine. These differences also influence the expression of immune system genes. For example, animals raised in isolation express more genes involved in inflammatory immune responses such as type I interferons. In contrast, genes associated with T cell function are expressed more highly in the pigs housed outdoors. Many dendritic cells in the intestinal lymphoid tissues produce retinoic acid, a metabolite of dietary vitamin A. The ability to synthesize retinoic acid is a property of many mucosal immune cells, and this appears to be a central regulator of mucosal immunity and homeostasis. Retinoic acid induces the expression of the intestinal homing receptors α4β7 and CCR9 on T cells and B cells. In association with transforming growth factor-β (TGF-β), it enhances T cell proliferation and cytotoxicity and is especially important in promoting Th2 and Treg differentiation in the intestine and in the homing of IgA+ B cells to mucosal surfaces (Chapter 38). Retinoic acid, therefore, normally suppresses Th1 and Th17 responses and favors tolerance to food antigens. Th17 cell development in the intestine is also affected by the microflora (Figure 22-5). Germ-free mice are deficient in IL-17. Segmented filamentous bacteria drive T cell development and stimulate Th17 production. SFBs also promote germinal center development, IgA production, and recruitment of intraepithelial lymphocytes. Because it is believed that Th17 cells may originate from T reg precursors, it is suggested that the microflora regulates the Th17-Treg switch. Changes in the composition of the intestinal microflora have been implicated in the development of allergies. The hygiene hypothesis suggests that a lack of exposure to certain commensal bacteria early in life affects the development of the immune system and increases an individual’s chances of developing allergic diseases. In support of this, it has been shown that children who develop allergic disease have different intestinal microflora than children who do not. Studies on piglets exposed to differing levels of intestinal colonization have shown that the complexity of the intestinal flora influences gene expression in the immune system. Animals with a limited intestinal flora expressed more genes involved in inflammation. Conversely, piglets with a more diverse intestinal flora expressed more genes related to T cell function. Alterations in the intestinal microflora also influence the development of autoimmune diseases such as rheumatoid arthritis, some mouse models of autoimmune arthritis, ankylosing spondylitis, insulin-dependent diabetes mellitus, and experimental autoimmune encephalitis (Chapter 35). The surface of the intestine is covered by a layer of enterocytes that form intercellular tight junctions and which thus form an effective barrier to both microbes and macromolecules (Figure 22-6). (Molecules larger than about 2 kDa are excluded.) Obviously, some aggressive invasive bacteria may damage this barrier and trigger local inflammatory and immune responses. There are two alternative routes by which organisms and macromolecules can penetrate the intact intestinal wall and be directed toward the intestinal lymphoid tissues. One route involves penetrating specialized epithelial cells (M cells) found in the epithelium directly over aggregates of lymphoid tissue or Peyer’s patches; the other involves dendritic cells that reside in the submucosa but extend their cytoplasmic processes between the enterocytes into the intestinal lumen. The tight junctions remain intact, but antigen samples can enter within the dendritic cell cytosol. This route provides a mechanism whereby noninvasive bacteria and macromolecules can be sampled and presented to nearby T cells. Peyer’s patches are the largest of the mucosal lymphoid tissues. A newborn calf normally has about 100 Peyer’s patches, and these may cover as much as half of the ileal surface. Collectively, therefore, the intestine contains more lymphocytes than the spleen. In ruminants and pigs, there are two types of Peyer’s patch that differ in location, structure, and functions. The ileocecal Peyer’s patches of ruminants are primary lymphoid organs, whereas the jejunal Peyer’s patches are secondary lymphoid organs (Figure 12-8). In lambs, the ileocecal patches increase in size from birth to 6 months of age and then regress, leaving only a small scar. In contrast, the jejunal patches persist throughout adult life and continue to play a major role in intestinal defense. Both types of Peyer’s patch consist of masses of lymphocytes arranged in follicles and covered with an epithelium that contains M cells. M cells are specialized epithelial cells involved in antigen transportation. They have microfolds (M) rather than microvilli on their surface (Figure 22-7). The mucus layer tends to thin out over Peyer’s patches so that the M cells protrude into the lumen. M cells phagocytose the macromolecules and microbes they encounter, but rather than destroy them, they transport the antigens to their underlying lymphoid tissue. M cells may transport soluble macromolecules such as IgA, small particles, and even whole organisms. (Some pathogens, such as salmonellae, Yersinia and Listeria species, M. tuberculosis, and the reoviruses may take advantage of the M cells and use them to gain access to the body.) The proportion of M cells in the follicle-associated epithelium varies from 10% in humans and mice to 50% in rabbits and to 100% in the terminal ileum of pigs and calves.
Immunity at Body Surfaces
The Body’s Microflora
The Superorganism
Controlling the Microflora
Exclusion of Commensals
Benefits of the Microflora
Exclusion of Pathogens
Regulation of B Cell Function
Regulation of T Cell Function
Dysbiosis and the Hygiene Hypothesis
Mucosal Lymphoid Tissues
Inductive Sites
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immunity at Body Surfaces
Only gold members can continue reading. Log In or Register to continue