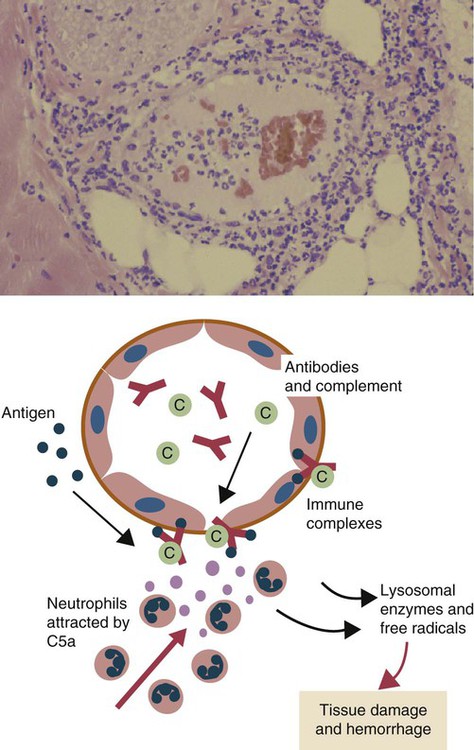
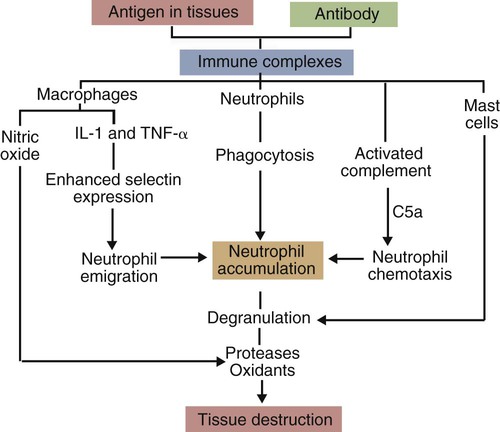
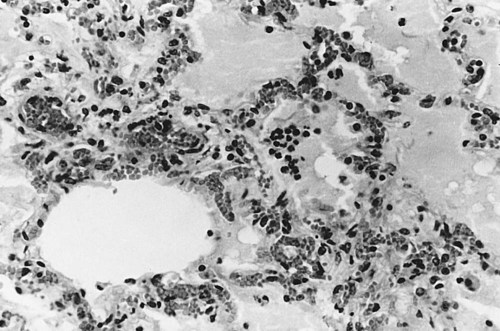
• When antigens and antibodies combine, they form immune complexes. Immune complexes can trigger severe inflammation when deposited in large amounts in tissues. This type of inflammation is classified as type III hypersensitivity. • Local deposition of immune complexes in the lungs following inhalation of antigenic dusts causes hypersensitivity pneumonitis. • Immune complexes formed in the bloodstream are deposited in the glomeruli of the kidney and cause membranoproliferative glomerulonephritis. • Type III hypersensitivity is a feature of many viral diseases, especially if the virus is not neutralized by antibodies and large amounts of immune complexes are generated as a result. The first events observed following antigen injection are neutrophil adherence to vascular endothelium followed by their emigration into the tissues. By 6 to 8 hours, when the reaction has reached its greatest intensity, the injection site is densely infiltrated by large numbers of these cells (Figure 30-1). As the reaction progresses, destruction of blood vessel walls results in hemorrhage and edema, platelet aggregation, and thrombosis. By 8 hours, mononuclear cells appear in the lesion, and by 24 hours or later, depending on the amount of antigen injected, they become the predominant cell type. Eosinophils are not a significant feature of this type of hypersensitivity. Immune complexes activate complement and generate the chemotactic peptide C5a (Figure 30-2). Neutrophils, attracted by C5a and mast cell–derived chemokines, emigrate from the blood vessels, adhere to immune complexes, and promptly phagocytose them. Eventually the immune complexes are digested and destroyed. During this process, however, proteases and oxidants are released into the tissues. When neutrophils attempt to ingest immune complexes attached to structures such as basement membranes, they secrete their granule contents directly into the surrounding tissues. Neutrophil proteases disrupt collagen fibers and destroy ground substances, basement membranes, and elastic tissue. Normally tissues contain antiproteinases that inhibit neutrophil enzymes. However, neutrophils can subvert these inhibitors by secreting OCl−. The OCl− destroys the inhibitors and allows tissue destruction to proceed. Blue eye is a condition seen in a small proportion of dogs that have been either infected or vaccinated with live canine adenovirus type 1 (see Figures 26-8 and 26-9). These animals develop an anterior uveitis leading to corneal edema and opacity. The cornea is infiltrated by neutrophils, and virus-antibody complexes are present in the lesion. Blue eye develops about 1 to 3 weeks after the onset of infection and usually resolves spontaneously as the virus is eliminated. The lesions of hypersensitivity pneumonitis consist of an acute alveolitis together with vasculitis and exudation of fluid into the alveolar spaces (Figure 30-3). The alveolar septa may be thickened, and the entire lesion is infiltrated with inflammatory cells. Since many of these cells are eosinophils and lymphocytes, it is obvious that the reaction is not a pure type III reaction. Nevertheless, examination of the lungs of affected cattle by immunofluorescence demonstrates deposits of immunoglobulin, complement, and antigen. In animals inhaling small amounts of an antigen over a long period, proliferative bronchiolitis and fibrosis may be observed. Clinically, hypersensitivity pneumonitis presents as a pneumonia occurring between 5 and 10 hours after acute exposure to grossly moldy hay. The animal may have difficulty breathing and develop a severe cough. In chronically affected animals, the dyspnea may be continuous. The most effective method of managing this condition is by removing the source of the antigen. Administration of steroids may be beneficial.
Immune Complexes and Type III Hypersensitivity
Local Type III Hypersensitivity Reactions
Blue Eye
Hypersensitivity Pneumonitis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Immune Complexes and Type III Hypersensitivity
Only gold members can continue reading. Log In or Register to continue