Fig. 5.1
Sections prepared by various preparation methods and stained with hematoxylin-eosin show that widely open sinusoids are clearly detected with “in vivo cryotechnique ” under normal blood circulation (IVCT-N; a, arrowheads) but collapsed with IVCT under ischemic conditions (IVCT-IC; c, arrowheads). Such sinusoidal collapse is more prominently seen with quick freezing of resected fresh specimens (FQF-1 min; b). Sinusoids with congested erythrocytes are dilated with IVCT after heart arrest (IVCT-HA; d, arrowheads). With perfusion -fixation followed by alcohol dehydration (PF-DH), sinusoids are opened without erythrocytes due to perfusion pressure (e, arrowheads). Sinusoids are mostly collapsed with immersion fixation followed by alcohol dehydration (IM-DH; f, arrowheads). Some nuclei of hepatocytes appear smaller with PF-DH and IM-DH (e, f; arrows). Asterisks: interlobular veins. Bars: 30 μm (The figure was reproduced from Ohno et al. [11])
IVCT also maintained the ultrastructures of living animal livers under normal blood circulation in tissue areas within several micrometers from the tissue surface [11]. Widely open sinusoids surrounded by fenestrated endothelial cell s were clearly observed, and Disse’s spaces were widely opened in some areas [11]. Processes of endothelial cells often extended into sinusoidal cavities, and bile canaliculi with microvilli and tight junction s were also open in living liver tissues. In contrast, sinusoids and Disse’s spaces were almost entirely collapsed by IM-DH.
Tissue resection, essential in the conventional QF method, may cause artifacts due to ischemia or anoxia on the mouse hepatic morphology. Tissue resection might cause cellular swelling due to rapid entry of extracellular components into the cytoplasm of ischemic cells [24, 25]. The severer collapse of sinusoids and decreased sizes of bile canaliculi observed with FQF may be attributable to these artificial swellings caused by tissue resection. “Intravital microscopy ” is a powerful approach to solve such technical problems [5, 7] and enables time-lapse observation of sinusoids and certain cells in vivo [8–10]. On the other hand, the dynamically changing morphology of cells and tissues can be examined on tissue sections with IVCT without artifacts induced by tissue resection, perfusion -fixation , and alcohol dehydration [26–28]. IVCT in combination with other techniques, including physiological monitoring or intravital microscopy, could be useful for immunohistochemical and ultrastructural studies of living liver tissues.
5.3 Distribution of Serum Proteins and Glycogen
Distribution of serum protein s in mouse liver was also clearly detected with IVCT [11]. Under normal blood circulation , immunostaining for IgG1 , Igκ, or albumin was observed along sinusoidal edges (Fig. 5.2). Igκ immunostaining also represents IgA and was found in bile canaliculi located in hepatic plates visualized by tight junction s immunopositive for ZO-1. Albumin immunoreactivity appeared as small dots and was observed in hepatocytes , presumably detected in Golgi areas. In tissue specimens prepared with PF-DH, immunostaining for serum proteins including Igκ and albumin was less clearly observed (Fig. 5.2).
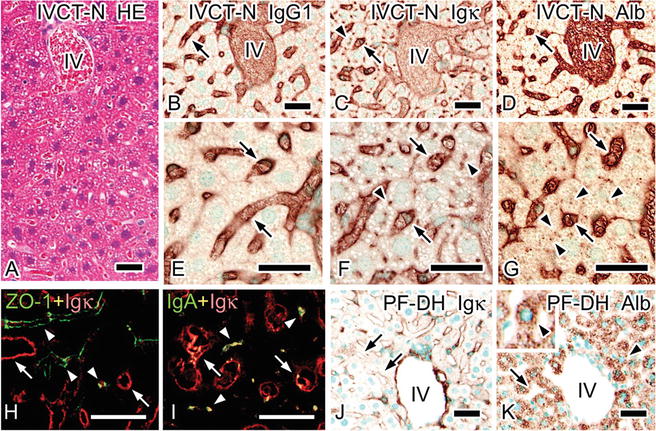

Fig. 5.2
Light micrographs of specimens prepared with “in vivo cryotechnique ” under normal circulation (IVCT-N; a–i) or perfusion-fixation followed by alcohol dehydration (PF-DH; j, k). Serial sections are stained with hematoxylin-eosin (HE; a) or immunostained for immunoglobulin G 1 heavy chain (IgG1 ; b, e), immunoglobulin kappa light chain (Igκ; c, f, j), or albumin (d, g, k). All serum protein s are clearly immunolocalized in sinusoids with IVCT-N (b–g, arrows), while immunostaining for Igκ and albumin is also detected among hepatic plates (f, g; arrowheads). Double immunofluorescence labeling for Igκ (h, i; red) and zonula occludens-1 (ZO-1; h; green) or immunoglobulin A (IgA; i; green) in cryosections prepared by IVCT-N shows that immunostaining for Igκ is detected between parallel ZO-1 immunostaining for tight junction s between hepatic plates (h; arrowheads) and is also seen together with that of IgA (i; arrowheads). Igκ or IgA is also immunolocalized in sinusoids (h, i; arrows). Immunoreactivity of Igκ or albumin is dramatically diminished in sinusoids with PF-DH (j, k; arrows), but some albumin in hepatocytes becomes condensed (k; arrowheads). IV interlobular veins. Bars: 30 μm (The figure was reproduced from Ohno et al. [11])
Soluble glycogen in hepatocytes was also well maintained with IVCT [11]. Abundant glycogen as visualized by strong periodic acid-Schiff (PAS ) staining was detected in hepatocytes located around centrilobular sinusoids and periportal zones with IVCT under normal blood circulation . In contrast, by FQF, the PAS staining was partially decreased in the peripheral cytoplasm of hepatocytes around centrilobular sinusoids. By PF-DH, such loss of PAS staining was more clearly observed in periportal zones, and PAS staining intensity in the cytoplasm was decreased in some hepatocytes located in the centrilobular areas. Decrease of PAS staining was worst in the liver specimens prepared by IM-DH.
Soluble molecules can be moved or lost in conventional tissue preparation methods such as PF-DH or IM-DH [29–32]. However, IVCT and QF do not use aqueous fixatives during specimen preparation and thus well-preserve soluble component s in the specimens [29, 31–33]. Signal molecules influencing hepatic microvasculature have been studied under pathological conditions [5–7, 34]. The nutritional state, such as the glycogen storage of hepatocytes , could be important for injury caused by ischemia -reperfusion stress during liver transplantation [35]. Therefore, the localization of soluble molecules would be effectively examined in living animal livers using IVCT.
5.4 Disrupted Cell Membrane Integrity
Because of the excellent preservation of soluble molecules in situ by the quick freezing followed by freeze-substitution, these methods also revealed that immunoreactivity of serum protein s is detected in some hepatocyte cytoplasm after tissue resection (Fig. 5.3) [11]. Interestingly, immunostaining for IgG1 and Igκ in the serial sections demonstrated that these serum proteins were colocalized in the same hepatocyte. The IgG1- and Igκ-immunopositive hepatocytes were also immunopositive for albumin , while some hepatocytes were immunopositive only for albumin (Fig. 5.3). Such diffuse immunostaining for the serum proteins was not detected with IVCT [11]. Since the molecular size of IgG is larger than that of albumin, the immunoreactivity of serum proteins in hepatocytes indicated size-dependent translocation of soluble serum protein s due to rapid permeability alterations of cell membranes caused by tissue resection. Actually, the disruption of the cell membranes is a hallmark of necrosis [36]. Soluble serum proteins as well as extrinsic dye would be easily translocated into hepatocytes upon necrosis which is characterized by size-dependent changes of cell membrane permeability following anoxia /ischemia . IVCT could be useful to analyze the integrity of hepatocyte cell membranes under physiological and pathological conditions in living animal liver .
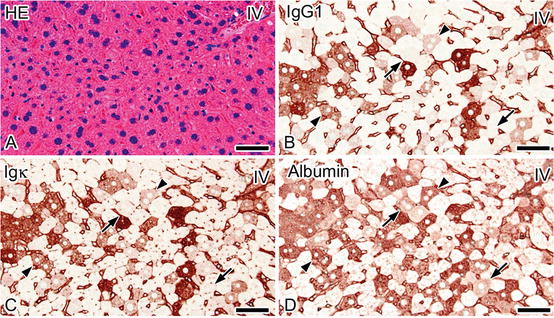

Fig. 5.3




Serial sections prepared by quick freezing of fresh tissues 5 min after resection (FQF-5 min) and stained with hematoxylin-eosin (HE; a) or immunostained for immunoglobulin G 1 heavy chain (IgG1 ; b), immunoglobulin kappa light chain (Igκ; c), or albumin (d) show that cytoplasm of some hepatocytes , indistinguishable with HE staining (a), is immunostained for IgG1, Igκ, and albumin (b–d; arrows, arrowheads). Immunodistribution of IgG1 and Igκ appears similar (b, c), whereas some albumin-immunopositive hepatocytes are not immunostained for IgG1 or Igκ (b–d; arrows). IV interlobular vein. Bars: 50 μm (The figure was reproduced from Ohno et al. [11])
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


