Fig. 29.1
Serial paraffin sections of antral follicles in the control (N; a–d, i–k, o–q, u–w) or mifepristone -induced polycystic ovary model (PCO; e–h, l−n, r–t, x–z) mouse ovaries prepared by in vivo cryotechnique and stained with hematoxylin-eosin (HE, a, e) or immunostained for albumin (b, f, i–n), immunoglobulin G (IgG1 , c, g, o–t), and IgM (d, h, u–z). The immunoreactivity of the three plasma proteins is clearly observed in the blood vessel s (BV; a–h, i, l, o, r, u, x, black arrowheads) of both control and PCO model mice. The albumin can be clearly detected in membrana granulosa (Gra) and antrum (Ant; b, f, j, k, m, n, white arrowheads). Immunoreactivity of IgG1 in the membrana granulosa is weaker than that in thecal layers and blood vessels in control mice (c, p, q, arrows). In contrast, immunoreactivity of IgG1 is very weak in the granulosa cell layer (g, s, arrows) and antrum (g, t, white arrowheads) in PCO model ovaries. The immunoreactivity for IgM in the interstitium (d, h, white arrowheads) is weaker than that in the blood vessels (d, h, u, x, black arrowheads), and no immunoreactivity is observed in the follicles of both control and PCO model mice (d, h, v, w, y, arrows). The regions marked with white rectangles in b–d and f–h are magnified in i–z. Bars, 40 μm (The figure is adapted from Zhou et al. [17])
IVCT along with double immunofluorescence labeling of collagen type IV , a marker for follicular and vascular basement membrane s , and multiple plasma proteins with intermediate molecular weights revealed altered distribution of the plasma proteins [17]. Immunoreactivity of IgG -Fc (150 kDa) was little in membrana granulosa of the antral follicles , in comparison with the thecal layers and blood vessel s (Fig. 29.2). In comparison with the thecal layers and blood vessels, almost no immunoreactivity of inter-alpha-trypsin inhibitor (ITI; 220 kDa) was observed in the membrana granulosa in the antral follicles (Fig. 29.2). The follicular basement membranes immunostained with collagen type IV bordered the prominent immunoreactivity decrease of IgG-Fc and ITI (Fig. 29.2). The immunostaining for fibrinogen (340 kDa) was largely restricted within the blood vessels, which was surrounded by the basement membranes immunopositive for collagen type IV (Fig. 29.2).
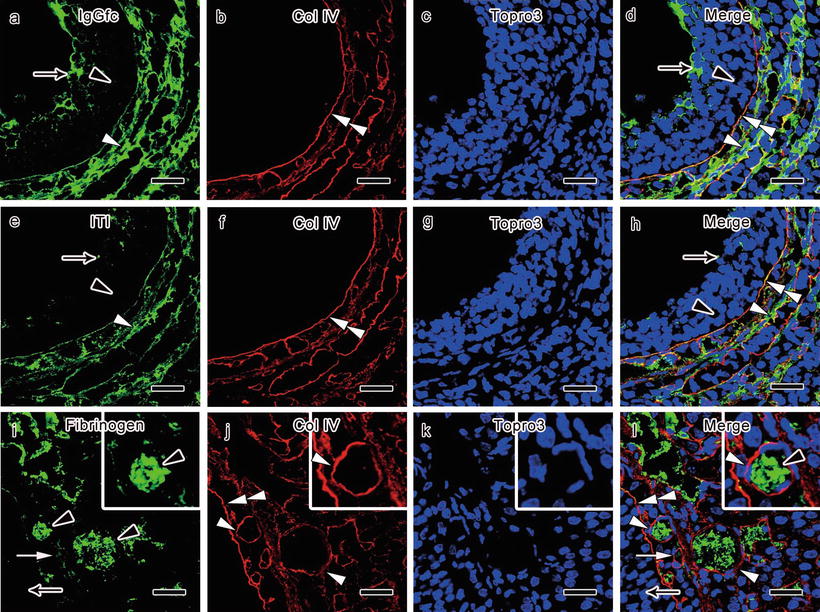

Fig. 29.2
Double immunostaining for collagen type IV (Col IV; b, d, f, h, j, l, red color) and immunoglobulin G -Fc (IgGfc ; a, d, green color), inter-alpha-trypsin inhibitor (IΤI; e, h, green color), and fibrinogen (i, l, green color) in PCO model mouse ovaries prepared with in vivo cryotechnique shows that little immunoreactivity of IgGfc (a, d, black arrowheads) is seen in the granulosa cell layer of the follicles , in comparison with the thecal layers and blood vessel s (a, d, white arrowheads). Some IgGfc immunoreactivity is detected in the antrum (a, d, arrows). Almost no immunoreactivity of ITI is seen in the granular cell layer (e, h, black arrowheads) and the antrum (e, h, arrows) of the follicles, in comparison with the thecal layers and blood vessels (e, h, white arrowheads). The decrease of IgGfc and ITI immunoreactivity is clearly bordered by the follicular basement membrane s immunopositive for Col IV (b, d, f, h, double white arrowheads). The immunoreactivity of fibrinogen is more clearly seen in the blood vessels of the thecal layers (i, l, arrowheads), while it is not detected inside the antral follicles (i, l, arrows). The alterations of fibrinogen immunoreactivity are mostly bordered by vascular basement membranes immunopositive for Col IV (j, l, white arrowheads) and also partly by the follicular basement membranes (j, l, double white arrowheads). The cellular nuclei are labeled with Topro3 (c, d, g, h, k, l, blue color). Bars, 20 μm (The figure is adapted from Zhou et al. [17])
In the PCO model mice examined with IVCT, the immunoreactivity of proteins with intermediate molecular weights appears decreased inside the follicles . These findings indicate that such permselective functions especially against proteins with intermediate molecular weights are enhanced in PCO. The impaired passage of middle-sized plasma proteins through follicular basement membrane s might be attributable to alterations of the extracellular matrix components around the developing follicles, since appropriate remodeling of extracellular matrices is essential for normal ovulation [33–35]. Although only a part of ITI binds to hyaluronic acid produced by granulosa cells to stabilize the cumulus extracellular matrix, the decrease of follicular ITI may also be involved in disturbed follicular expansion and ovulation [36–38]. On the other hand, high fibrinogen (340 kDa) immunoreactivity was bordered at the vascular endothelium which was surrounded by basement membranes immunopositive for collagen type IV (Fig. 29.2) [17]. In normal mouse ovaries, fibrinogen passed through the basement membranes into the interstitium [15]. The endothelium is considered to be one of the important sites for the BFB permselectivity [15, 16]. Dynamic cellular rearrangements, partly regulated by cell–cell adhesion, play essential roles in morphological changes during follicular development [39, 40]. The tight junction is located on the apical side of epithelial and endothelial cell s to completely seal their intercellular spaces [41, 42]. Downregulation of claudin 5 is caused by VEGF inhibition in the endothelium of the thecal vasculature [43]. The upregulation of claudin may contribute to the enhanced permselectivity of endothelial cells, given that the ovarian blood flow and VEGF expression in blood circulation are significantly increased in the PCO syndrome [32].
Since the structural components of basement membrane s are responsible for the permselectivity [44, 45], collagen type IV α chains were immunohistochemically examined to reveal any changes in the major components of the basement membranes [17, 46, 47]. However, immunohistochemical analyses of the basement membrane components, including collagen type IV α2, α4, and α5 chains, in control and PCO model ovaries revealed that there was no detectable change of the basement membrane components [17]. In addition, it was previously reported in PCO that there were few ultrastructural changes in follicular basement membranes [48]. Further analyses on other molecular components of basement membranes, such as perlecan, nidogens, and laminins, and ultrastructures of the follicular basement membranes using IVCT would be useful, since the conventional preparation methods cause shrinkage artifacts in basement membranes at an ultrastructural level [49].
29.4 Concluding Remarks
With IVCT, morphological changes and the immunoreactivity of plasma proteins were clearly observed in living mouse ovaries of the PCO model. Enlarged blood vessel s were observed in the ovaries of the PCO model mice prepared with IVCT, indicating increased blood flow into the ovaries. In addition, in the PCO model ovaries, follicular cysts with degenerative membrana granulosa were formed. Plasma proteins with intermediate molecular weights, including IgG1 , ITI, and fibrinogen , appeared to be strictly bordered at the BFB in the PCO model mice, although the distribution of plasma proteins with low and high molecular weights, including albumin and IgM , was similar in the PCO model and normal mice. IVCT revealed that the PCO model ovaries are characterized by the increased blood circulation and higher selectivity of plasma protein permeation through the BFB. The pathogenesis and pathophysiology of PCO syndrome might by influenced by the alterations of hemodynamic condition s and permselectivity of BFB.



