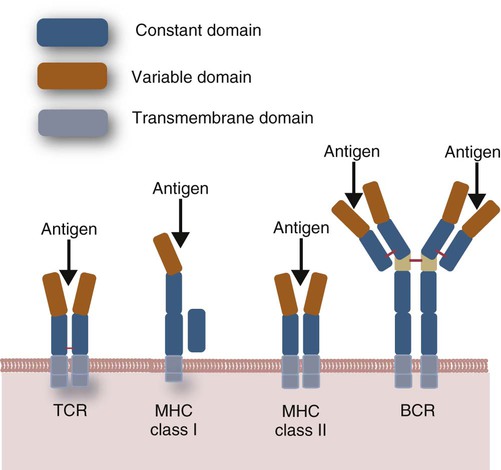
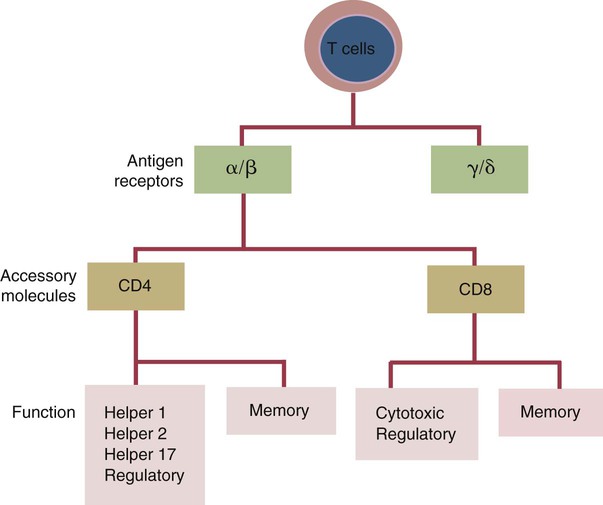
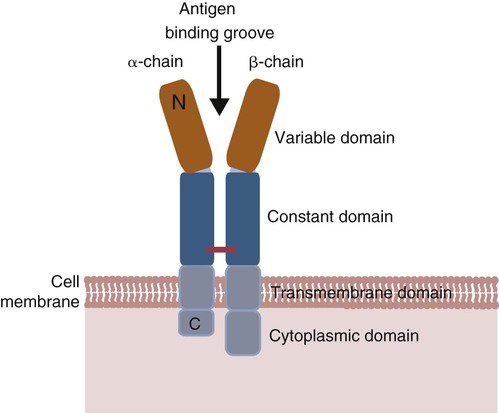
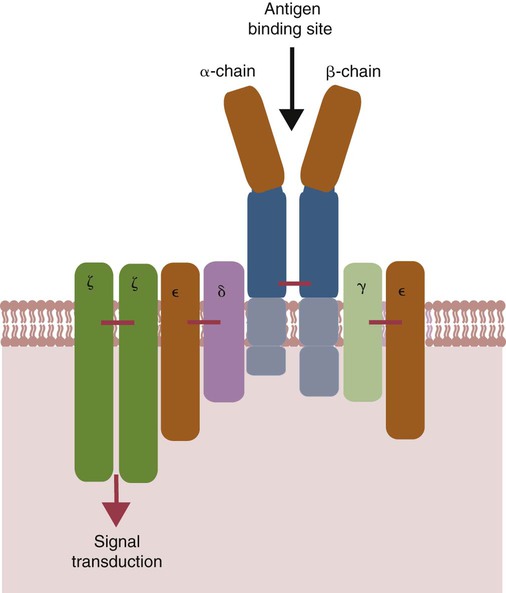
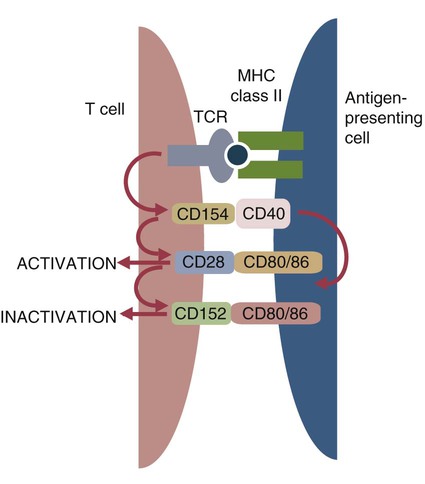
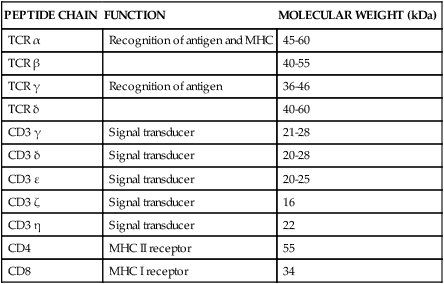
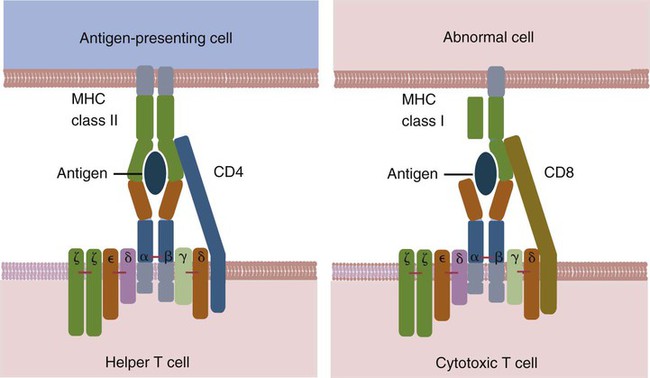
• Helper T cells express antigen receptors (TCRs) consisting of paired peptide chains, either α and β or γ and δ. • These paired chains form antigen-binding receptors whose ligands are peptides linked to major histocompatibility complex (MHC) molecules on antigen-presenting cells. • The antigen-binding chains of the TCR connect to a complex signal transducing component called CD3. • Each TCR is also associated with either CD4 or CD8. CD4 binds to MHC class II molecules on antigen-presenting cells. CD8 binds to MHC class I molecules expressed on all nucleated cells. • To respond to antigens, T cells must bind to antigenic peptides linked to MHC molecules. They must also receive co-stimulation from cytokines and other molecules. • The multiple signals sent by an antigen-presenting cell are communicated to a T cell through an immunological synapse. • There are three major subpopulations of helper T cells. Th1 cells are stimulated by interleukin-12 (IL-12) and secrete interferon-γ (IFN-γ) in response. They generally promote cell-mediated responses. • Th2 cells secrete IL-4, 1L-13, and IL-10. They generally promote antibody responses. • Th17 cell development is stimulated by IL-6, TGF-β, and IL-23. They secrete IL-17 and promote neutrophil-mediated inflammation. • α/β Helper T cells are the predominant T cells in most mammals. γ/δ Helper T cells are mainly confined to the intestinal wall in humans but are the predominant circulating T cells in young ruminants and pigs. Proteins belonging to the immunoglobulin superfamily play key roles in immunity. The members of this superfamily all contain at least one immunoglobulin domain. In a typical immunoglobulin domain, the peptide chains weave back and forth to form a pleated sheet that folds into a sandwich-like structure. Immunoglobulin domains were first identified in antibody molecules (immunoglobulins). They have since been found in many other proteins, and collectively these proteins form the immunoglobulin superfamily. The superfamily includes some proteins with multiple immunoglobulin domains and some with only a single domain. Important proteins with multiple domains include the B cell antigen receptors (BCRs), the T cell antigen receptors (TCRs), and the MHC class I and II molecules (Figure 14-1). All of the members of this superfamily are receptors, most are found on cell surfaces, and none has enzymatic activity. Many cellular responses are triggered by interactions between two different members of the superfamily as, for example, between TCR and MHC molecules. Each T cell has about 30,000 identical antigen receptors (TCRs) on its surface. Each TCR is a complex structure containing multiple glycoprotein chains. Two of these chains are paired to form the antigen-binding site; the other chains transmit the signal generated by antigen binding to the cell. Two different types of TCR have been identified based on the paired peptide chains used for antigen binding (Figure 14-2). One type employs γ and δ (γ/δ) chains. The other employs α and β (α/β) chains. In humans, mice, and probably most nonruminants, 90% to 99% of T cells use α/β receptors. In calves, lambs, and piglets in contrast, up to 66% of T cells may use γ/δ receptors. The four antigen-binding chains (α, β, γ, δ) are similar in structure, although they differ in size. Thus the α chain is 43 to 49 kDa, the β chain is 38 to 44 kDa, the γ chain is 36 to 46 kDa, and the δ chain is 40 kDa. Size differences are due to variations in glycosylation. Each of these chains is formed from four domains (Figure 14-3). The N-terminal domain contains about 100 amino acids whose sequence varies greatly among cells. This is therefore called the variable (V) domain. The second domain contains about 150 amino acids. Its amino acid sequence does not vary, so it is called the constant (C) domain. The third, very small domain consists of 20 hydrophobic amino acids passing through the T cell membrane. The C-terminal domain within the cytoplasm of the T cell is only 5 to 15 amino acids long. The paired chains are joined by a disulfide bond between their constant domains to form a stable heterodimer. As a result, the two V domains form a groove in which antigens and MHC molecules bind. The precise shape of this antigen-binding groove varies among different TCRs because of the variable amino acid sequences in the V domains. The specificity of the binding between a TCR and an antigen is determined by the shape of the groove formed by the V domains. The binding of antigen to the TCR sends a signal to trigger the T cell response. The two antigen-binding chains of each TCR are associated with a cluster of signal transducing proteins called the CD3 complex (Figure 14-4). The CD3 complex consists of five chains (γ, δ, ε, ζ, and η) (Table 14-1) arranged as three dimers γ-ε, δ-ε, and either ζ-ζ or ζ-η. The TCR β chain is linked to the γ-ε dimer, and the TCR α chain is linked to the δ-ε dimer. About 80% of α/β TCRs contain a ζ-ζ homodimer, so that the complete complex consists of αβ-γε-δε-ζζ. The remaining 20% contain ζ-η heterodimers (they therefore consist of αβ-γε-δε-ζη). Table 14-1 Two other proteins closely associated with the TCR are CD4 and CD8. CD4 is a single-chain glycoprotein of 55 kDa, and CD8 is a dimer of 68 kDa. (One chain of CD8 is called α, the other is β. In humans, pigs, mice, and cats, CD8 is an α-β heterodimer or, less commonly, an α-α homodimer.) Both CD4 and CD8 are members of the immunoglobulin superfamily. The presence of CD4 or CD8 determines the class of MHC molecule that is recognized by the T cell (Figure 14-5). For example, CD4, found only on helper T cells, binds MHC class II molecules on antigen-presenting cells. CD8, in contrast, is found only on cytotoxic T cells and binds MHC class Ia molecules on virus-infected or other abnormal cells. CD4 and CD8 enhance TCR signal transduction 100-fold when they cross-link to an MHC molecule on an antigen-presenting cell. Several additional receptors must be stimulated in order to activate T cells. CD40 is a receptor expressed on antigen-presenting cells. Its ligand is CD154, a protein expressed on helper T cells several hours after their TCRs have bound antigen (Figure 14-6). When CD154 and CD40 bind, signals are sent in both directions. The signal from the antigen-presenting cell to the T cell causes it to express a receptor called CD28. The signal from the T cell to the antigen-presenting cell stimulates it to express either CD80, or CD86. CD40-CD154 signaling also stimulate the antigen-presenting cell to secrete multiple cytokines, including interleukin-1 (IL-1), IL-6, IL-8, IL-12, CCL3, and tumor necrosis factor-α (TNF-α).
Helper T Cells and Their Response to Antigen
Immunoglobulin Superfamily
T Cell Antigen Receptor
Antigen-Binding Component
Signal Transduction Component
CD3 Complex
PEPTIDE CHAIN
FUNCTION
MOLECULAR WEIGHT (kDa)
TCR α
Recognition of antigen and MHC
45-60
TCR β
40-55
TCR γ
Recognition of antigen
36-46
TCR δ
40-60
CD3 γ
Signal transducer
21-28
CD3 δ
Signal transducer
20-28
CD3 ε
Signal transducer
20-25
CD3 ζ
Signal transducer
16
CD3 η
Signal transducer
22
CD4
MHC II receptor
55
CD8
MHC I receptor
34
CD4 and CD8
Co-stimulators
Co-stimulatory Receptors
CD40-CD154 Signaling
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Helper T Cells and Their Response to Antigen
Only gold members can continue reading. Log In or Register to continue