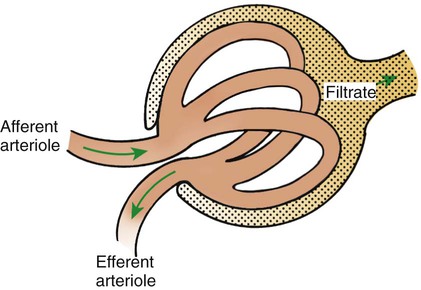
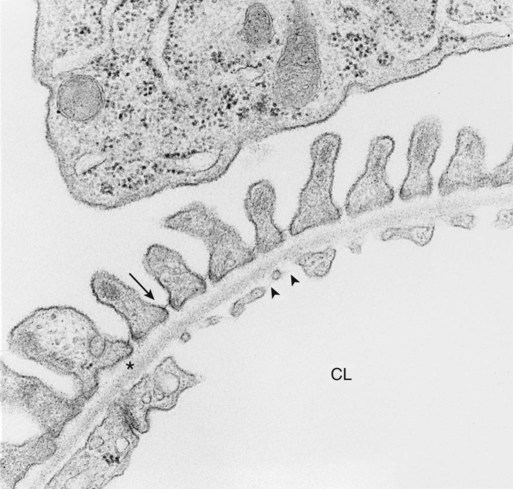
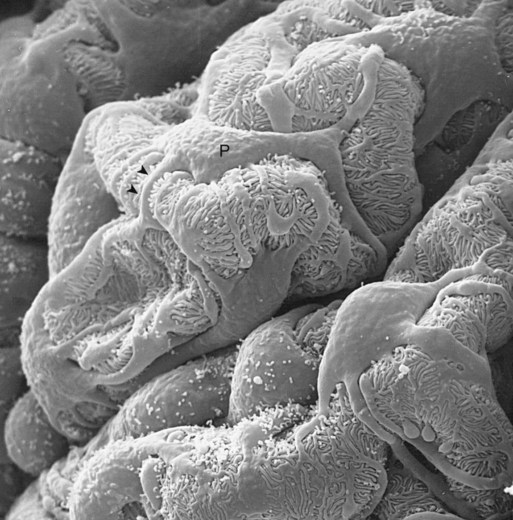
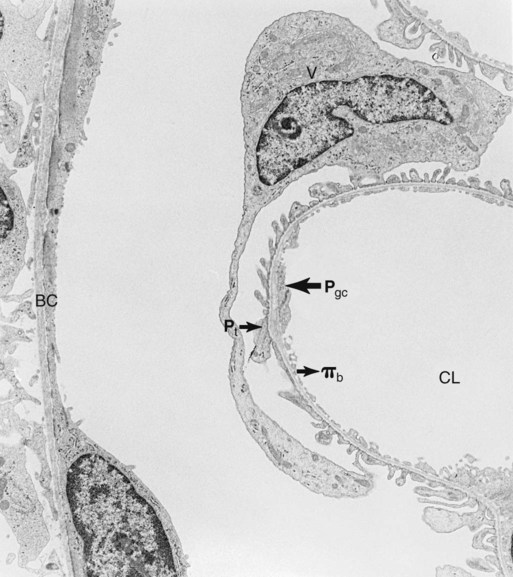
1. Introduction to the physiology of the kidney. 2. The glomerulus filters the blood. 3. The structure of the glomerulus allows efficient, selective filtration. 4. Glomerular filtration rate is determined by the mean net filtration pressure, permeability of the filtration barrier, and area available for filtration. 5. The filtration barrier is selectively permeable. 6. Glomerular filtration rate is regulated by systemic and intrinsic factors. 7. Glomerular filtration rate is measured by determining the plasma clearance rate of a substance. These myriad functions are accomplished by an extensive variety of cell types, each with specific responses to direct and indirect signals, arranged in a particular pattern to form the functional unit of the kidney, the nephron. The nephron is composed of the glomerulus, where the blood is filtered, and its associated renal tubule segments, where filtered substances are absorbed from, and plasma components are secreted into, the tubule fluid. In the renal cortex the nephrons merge into the collecting duct system, which traverses the kidney and empties into the renal pelvis. Figure 41-1 provides an overview of the anatomical arrangement of nephrons within the kidney and the major functions of the nephron and collecting duct segments. The glomerular tuft is composed of a network of capillaries (Figure 41-2). In mammals, renal arterial blood flows to the afferent arteriole, which divides into numerous glomerular capillaries. The capillaries anastomose to form the efferent arteriole, which conducts the filtered blood away from the glomerulus (Figure 41-3). Avian kidneys contain both mammalian-type and reptilian-type nephrons; in glomeruli of reptilian-type nephrons, the capillaries have few branches. The structure of the glomerular capillaries is important in determining the rate and selectivity of glomerular filtration. The wall of the capillary consists of three layers: the capillary endothelium, the basement membrane, and the visceral epithelium (Figure 41-4). The capillary endothelium is a single layer of very thin cells that faces the blood in the capillary lumen. Endothelial fenestrae (“windows”) are transcellular pores that conduct water and noncellular components in the blood to the second layer of the glomerular capillary wall, the glomerular basement membrane (GBM). The GBM is acellular and composed of various glycoproteins, primarily laminins, type IV collagens, nidogens, and the heparin sulfate proteoglycans, agrin in mature animals and perlecan in developing glomeruli. Compared to other basement membranes, the GBM is thicker and contains distinct glycoprotein isoforms. The GBM has three layers, created during development by the fusion of the basement membranes of the endothelial and epithelial cell layers. The three layers are named according to their density and relative position. As shown in Figure 41-4, the lamina densa (dense layer) is relatively dark because it is relatively resistant to the passage of electrons when viewed with a transmission electron microscope. The lamina densa is composed of tightly packed glycoprotein fibrils. It is sandwiched between the lamina rara interna (inside thin layer) on the endothelial side of the GBM and the lamina rara externa (outside thin layer) on the epithelial side of the GBM. The laminae rarae are composed of a loose network of glycoprotein fibrils. The third compartment of the glomerular capillary wall is the visceral epithelium, which is a layer of intricate, interlocking cells called podocytes. Numerous long, narrow extensions, the primary and secondary foot processes, interdigitate with foot processes from other podocytes and wrap around the individual capillaries (Figure 41-5). The epithelial slit diaphragm spans between adjacent foot processes (see Figure 41-4). The transmembrane protein, nephrin, is a critical component of this structure; the extracellular domain of nephrin molecules extending from adjacent foot processes interacts to form the slit diaphragm. The glomerular capillary wall creates a barrier to the forces favoring and opposing filtration of the blood. The forces favoring filtration—that is, movement of water and solutes across the glomerular capillary wall—are the hydrostatic pressure of the blood within the capillary and the oncotic pressure of the fluid in Bowman’s space (the ultrafiltrate). Normally, the oncotic pressure of the ultrafiltrate is inconsequential because medium– to high–molecular-weight proteins are not filtered. Therefore the main driving force for filtration is the glomerular capillary hydrostatic pressure. Forces opposing filtration are the plasma oncotic pressure within the glomerular capillary and the hydrostatic pressure in Bowman’s space. Figure 41-6 illustrates the direction and magnitude of these forces under normal conditions.
Glomerular Filtration
Introduction to the Physiology of the Kidney
The Structure of the Glomerulus Allows Efficient, Selective Filtration
Glomerular Filtration Rate Is Determined by the Mean Net Filtration Pressure, Permeability of the Filtration Barrier, and Area Available for Filtration
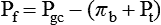
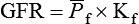
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Glomerular Filtration
Only gold members can continue reading. Log In or Register to continue
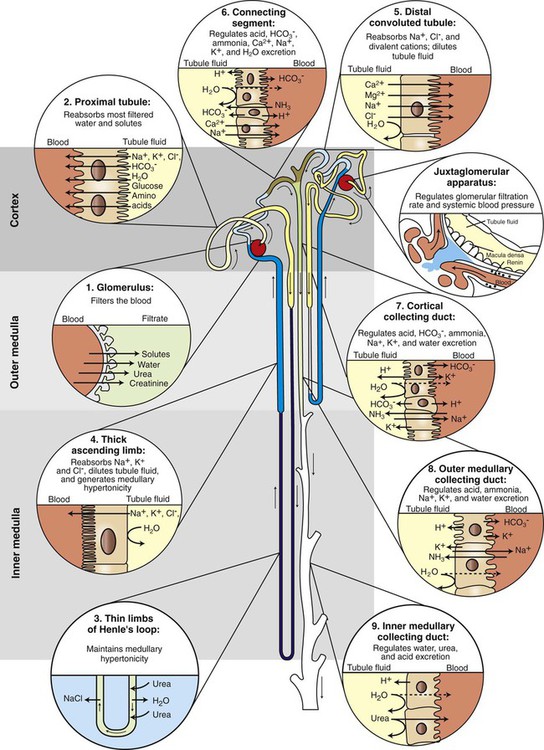
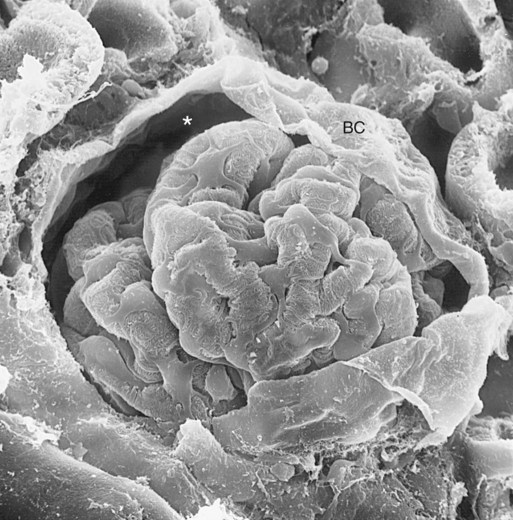
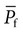 ), the permeability of the filtration barrier, and the surface area available for filtration. The permeability of the filtration barrier is determined by the structural and chemical characteristics of the glomerular capillary wall. The product of the filtration barrier permeability and its surface area is the ultrafiltration coefficient (Kf). Thus the combined effects of the determinants of GFR are mathematically represented by the following equation:
), the permeability of the filtration barrier, and the surface area available for filtration. The permeability of the filtration barrier is determined by the structural and chemical characteristics of the glomerular capillary wall. The product of the filtration barrier permeability and its surface area is the ultrafiltration coefficient (Kf). Thus the combined effects of the determinants of GFR are mathematically represented by the following equation: