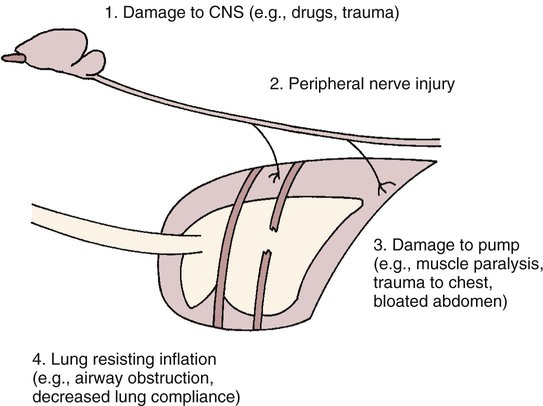
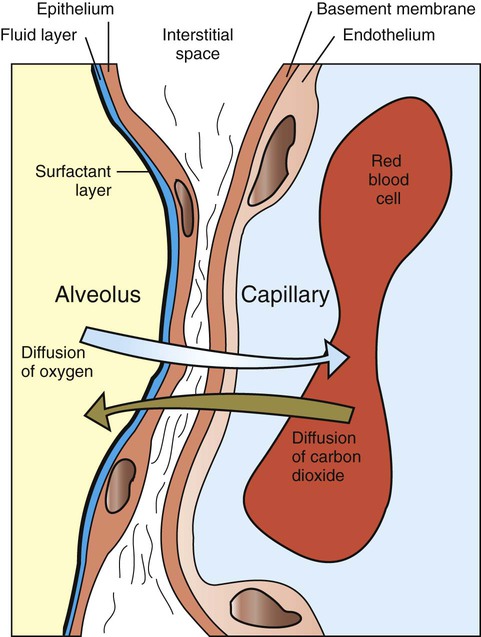
1. The composition of a gas mixture can be described by the fractional composition or partial pressure. 2. Alveolar gas composition is determined by alveolar ventilation and the exchange of oxygen and carbon dioxide. 3. Exchange of oxygen and carbon dioxide between the alveolus and pulmonary capillary blood occurs by diffusion. 4. The exchange of gases between the tissues and blood also occurs by diffusion. 5. The amount of alveolar ventilation in relation to pulmonary capillary blood flow—the 6. The composition of the systemic arterial blood is determined by the composition of the capillary blood that drains each alveolus. 7. Right-to-left vascular shunts allow blood to bypass ventilated lung. 8. Part of each breath ventilates dead-space and does not participate in gas exchange. 9. Arterial oxygen (PaO2) and carbon dioxide (PaCO2) tensions are measured to evaluate gas exchange. Understanding gas exchange requires an understanding of the measurement of gas composition and the forces causing gas movement within the lungs, blood, and tissues. For convenience, physiologists use many abbreviations when describing gas exchange (Table 47-1). Air contains 21% oxygen (the fraction of oxygen in inspired air, FiO2, is 0.21). High in the Andes Mountains, the air still contains 21% oxygen, but visitors to those altitudes notice the lack of oxygen. Clearly, therefore, it is not only the fraction of oxygen that is important for gas exchange; the hypoxia at high altitude is a result of the low oxygen partial pressure that is a consequence of the low barometric pressure. At this lower barometric pressure, the oxygen molecules are less densely packed, and therefore the partial pressure of oxygen (PO2) in the air is decreased. It is this partial pressure (also called tension) that is important in gas transfer. TABLE 47-1 Common Abbreviations Used in Gas Exchange PO2 decreases at higher altitudes because barometric pressure decreases. Alveolar hypoventilation, a decrease in alveolar ventilation in relation to carbon dioxide production, elevates PACO2 and decreases PAO2. Figure 47-1 shows the causes of alveolar hypoventilation. It occurs when (1) the central nervous system is depressed by drugs or injury, (2) there is injury to the phrenic nerve that supplies the diaphragm, (3) there is damage to the thorax and respiratory muscles, (4) there is severe airway obstruction (e.g., in exercising horses with laryngeal hemiplegia), or (5) there is lung disease that severely decreases lung compliance. In the lung the barrier separating air and blood (x) is less than 1 µm thick (Figure 47-2). However, although thin, this barrier includes a layer of liquid and surfactant lining the alveolar surface; an epithelial layer, usually formed by type I epithelial cells; a basement membrane; variable-thickness interstitium; and a layer of endothelium. In addition to moving gases through this air-blood barrier, diffusion also moves gases within the plasma, allowing oxygen to gain access to erythrocytes and hemoglobin. Normally, in the resting animal, equilibration between alveolar and capillary oxygen tensions occurs within 0.25 second, approximately one third of the time the blood is in the capillary (Figure 47-3). During strenuous exercise, muscles extract a large amount of oxygen from the blood, so the mixed venous blood returning to the lung contains little oxygen. In addition, during exercise the cardiac output is high, and the velocity of blood flow through the capillaries is rapid. More oxygen must therefore be transferred in less time than in the resting animal. Under these strenuous conditions, diffusion equilibrium may not occur, and the oxygen tension of blood leaving the lung and entering the systemic arteries (PaO2) may decrease during intense exercise. This exercise-associated hypoxemia is observed in racing Thoroughbred horses.
Gas Exchange
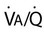 ratio—determines the adequacy of pulmonary gas exchange.
ratio—determines the adequacy of pulmonary gas exchange.
The Composition of a Gas Mixture Can Be Described by the Fractional Composition or Partial Pressure
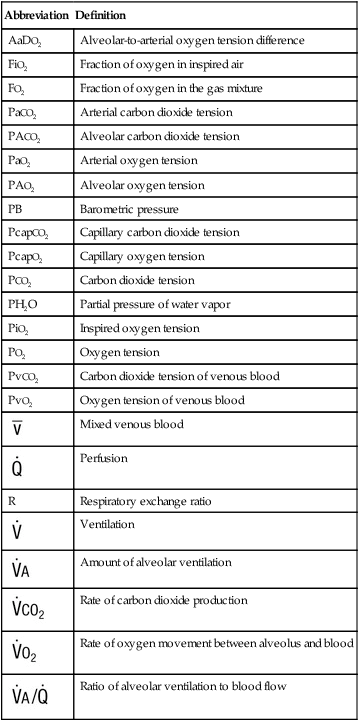
Abbreviation
Definition
AaDO2
Alveolar-to-arterial oxygen tension difference
FiO2
Fraction of oxygen in inspired air
FO2
Fraction of oxygen in the gas mixture
PaCO2
Arterial carbon dioxide tension
PACO2
Alveolar carbon dioxide tension
PaO2
Arterial oxygen tension
PAO2
Alveolar oxygen tension
PB
Barometric pressure
PcapCO2
Capillary carbon dioxide tension
PcapO2
Capillary oxygen tension
PCO2
Carbon dioxide tension
PH2O
Partial pressure of water vapor
PiO2
Inspired oxygen tension
PO2
Oxygen tension
PvCO2
Carbon dioxide tension of venous blood
PvO2
Oxygen tension of venous blood
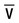
Mixed venous blood
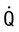
Perfusion
R
Respiratory exchange ratio
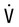
Ventilation
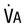
Amount of alveolar ventilation
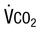
Rate of carbon dioxide production
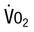
Rate of oxygen movement between alveolus and blood
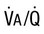
Ratio of alveolar ventilation to blood flow
Alveolar Gas Composition Is Determined by Alveolar Ventilation and the Exchange of Oxygen and Carbon Dioxide
Exchange of Oxygen and Carbon Dioxide Between the Alveolus and Pulmonary Capillary Blood Occurs by Diffusion
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Gas Exchange
Only gold members can continue reading. Log In or Register to continue
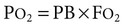
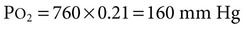
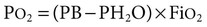
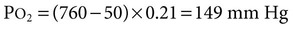
 ) in relation to the amount of alveolar ventilation (
) in relation to the amount of alveolar ventilation ( ):
):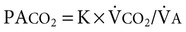
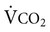 increases, as occurs during exercise,
increases, as occurs during exercise, 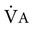 must also increase if PA
must also increase if PA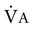 does not increase sufficiently, PA
does not increase sufficiently, PA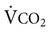 remains constant and
remains constant and 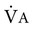 halves, PA
halves, PA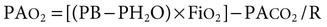
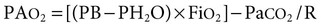
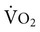 ) is determined by the physical properties of the gas (D), the surface area available for diffusion (A), the thickness of the air-blood barrier (x), and the driving pressure gradient of the gas between the alveolus and capillary blood (PA
) is determined by the physical properties of the gas (D), the surface area available for diffusion (A), the thickness of the air-blood barrier (x), and the driving pressure gradient of the gas between the alveolus and capillary blood (PA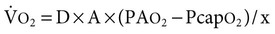
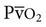 ) of approximately 40 mm Hg. The driving pressure gradient of 60 mm Hg (100−40) causes rapid diffusion of oxygen into the capillary, where it combines with hemoglobin. Hemoglobin takes up oxygen from the plasma and helps maintain the gradient for oxygen diffusion.
) of approximately 40 mm Hg. The driving pressure gradient of 60 mm Hg (100−40) causes rapid diffusion of oxygen into the capillary, where it combines with hemoglobin. Hemoglobin takes up oxygen from the plasma and helps maintain the gradient for oxygen diffusion.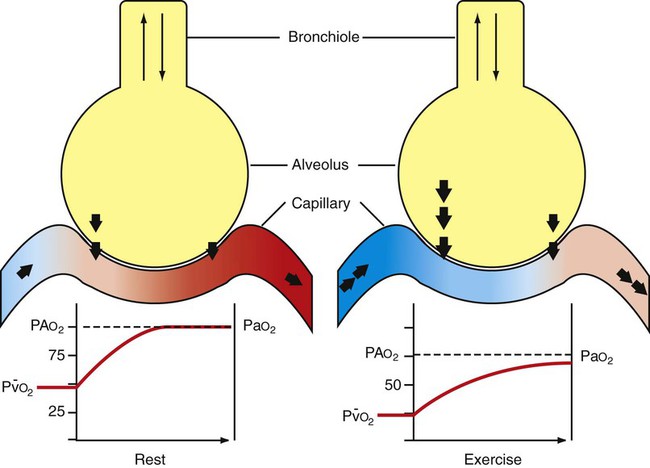
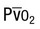 ) is approximately 40 mm Hg, and blood and air equilibrate rapidly. In the exercising animal, mixed venous oxygen tension is low, and even though oxygen fluxes are high, the blood has not equilibrated with the alveolar oxygen tension before it leaves the alveolus. PA
) is approximately 40 mm Hg, and blood and air equilibrate rapidly. In the exercising animal, mixed venous oxygen tension is low, and even though oxygen fluxes are high, the blood has not equilibrated with the alveolar oxygen tension before it leaves the alveolus. PA