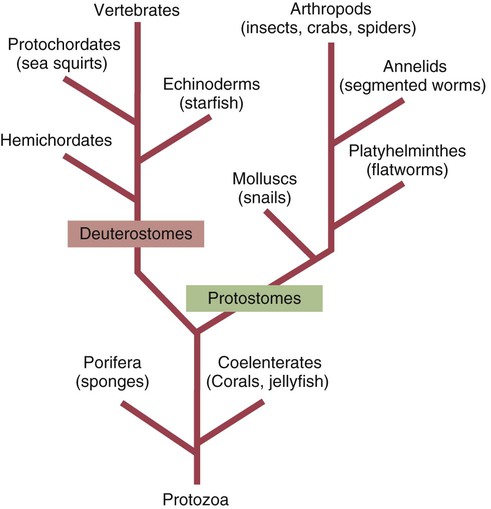
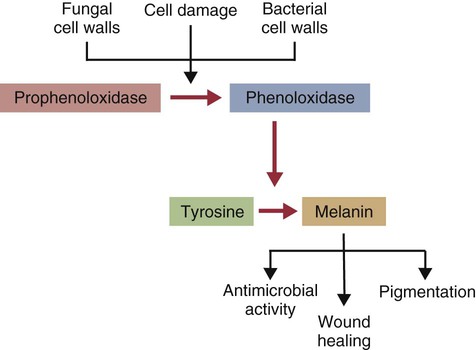
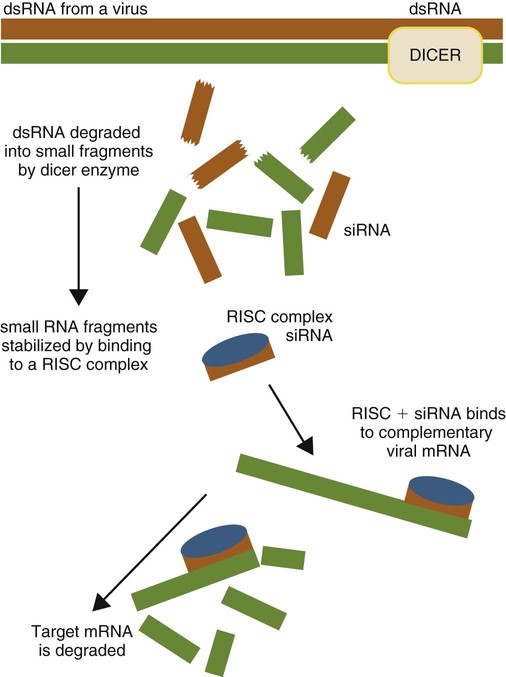
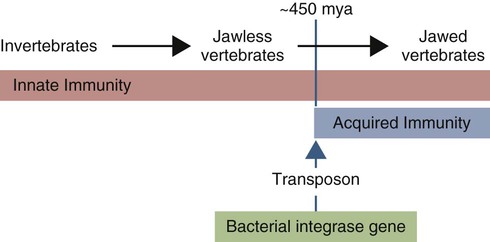
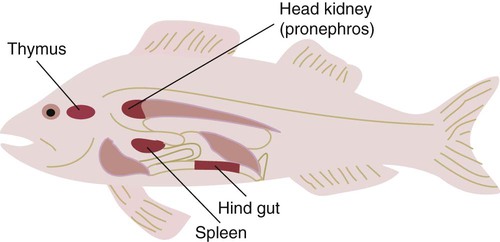
• Invertebrates rely exclusively on the use of innate immune mechanisms to protect themselves against infectious agents. • The jawless fish mainly rely on innate immunity, although they also possess a remarkably complex and diverse antigen-binding receptor system. • Cartilaginous fish are the first vertebrates to utilize an adaptive immune system. • It is suggested that the appearance of adaptive immunity occurred relatively suddenly during the evolutionary process with the incorporation into the fish genome of a microbial transposon containing recombinase genes. • Jawed fish and all the more developed vertebrates possess both antibody and cell-mediated immune systems, although the details differ between species. • The major chicken immunoglobulin is called IgY because it is structurally different from mammalian IgG. Invertebrates are classified based on the presence of a body cavity or coelom (Figure 40-1). The acoelomates include the sponges and coelenterates (jellyfish and sea anemones). The coelomates evolved further into two major lines. One line includes the annelids, mollusks, and arthropods, collectively called the protostomes. The other line, including the echinoderms, protochordates, and chordates, is called the deuterostomes. It is from deuterostome-like ancestors that the vertebrates evolved. Invertebrates rely exclusively on physical barriers and innate immune defenses to exclude microbial invaders. This system, found in arthropod hemolymph, consists of multiple enzymes that, when activated, generate a cascade of proteases leading to the production of the inert polymeric pigment melanin (Figure 40-2). The system is activated by the interaction of bacterial and fungal LPSs, peptidoglycans, and glucans with hemocytes. Activation also occurs through cuticular and hemolymph proteases. The proPO system generates phenoloxidase, a sticky enzyme that binds to foreign surfaces. This enzyme acts on tyrosine and dopamine to generate melanin and deposit it around inflammatory sites. Melanin polymer is deposited in the tissues surrounding invaders to form an impermeable barrier that blocks their nutrient uptake. Oxidizing agents and other antimicrobial molecules are also generated during melanin synthesis. The intracellular RNA interference pathway (RNAi) is a gene-silencing system that appears to have evolved to prevent viruses from replicating within infected cells. It is especially important as a defense system in invertebrates (Figure 40-3). RNA normally occurs only in a single-stranded (ss) form. Long segments of double-stranded RNA (dsRNA) are not present in healthy eukaryotic cells, but they do occur if a cell is infected by RNA viruses. When a virus-infected cell produces dsRNA, it is rapidly degraded into many short fragments by an enzyme called dicer. These fragments, or small-interfering RNAs (siRNAs) are then stabilized by a protein complex called the RNA-induced silencing complex (RISC). Half of these siRNAs will be complementary to the viral messenger RNAs (mRNAs) and as a result can serve as templates and bind them. Once these viral mRNAs have been captured by binding to the RISC complex, they are degraded rapidly, and viral replication effectively blocked. The adaptive immune system depends on possession of two key antigen receptor systems, the TCR and the B cell receptor (BCR). Both require the rearrangement of V, D, and J gene segments to form functional, antigen-binding receptors. Invertebrates and cyclostomes cannot rearrange these genes, but cartilaginous and bony fish can. Sometime during the 100 million years between the divergence of jawless and jawed vertebrates and the emergence of cartilaginous and bony fish, about 450 Mya, the enzymatic machinery needed for the recombination of V gene segments emerged. The mechanism of this sudden appearance is unknown. It has been suggested, however, that a transposon carrying the precursors of the recombinase-activating genes RAG1 and RAG2 (most likely a bacterial integrase) was successfully inserted into an immunoglobulin superfamily V-like gene within the germline of the early jawed vertebrates (Figure 40-5). As a result, the immunoglobulin gene could be expressed only after splicing mediated by the RAG enzymes. Thus emerged, in a major evolutionary leap, the ability to generate antigen-binding sites and functional immunoglobulins. This, for the first time, permitted animals to respond specifically to previously encountered antigens. The advantages of this new “improved” system were such that it is now a feature of all jawed vertebrates. This did not, of course, result in discarding of the innate immune defenses. Lectins, the complement system, and the NK cell system remain essential components of vertebrate immunity. It is also important to point out that adaptive immunity did not confer invincibility to infectious agents. It simply made life more difficult for them and conferred an incremental selective advantage on animals with such defenses. The selective advantage of adaptive immunity came with an attendant cost—the potential for autoimmune disease. Both cartilaginous and bony fish can mount adaptive immune responses and have a complete set of lymphoid organs except for a bone marrow (Figure 40-6). They have a thymus located just above the pharynx that arises from the first gill arches. In immature fish, small pores lead from the pharynx to the thymus, suggesting that it may be stimulated directly by antigens in the surrounding water. Thymectomy in fish can lead to prolongation of allograft survival and reduced antibody responses. Antibodies or antigen-binding cells may be detected in the thymus during an immune response, suggesting that it contains both T-like and B-like cells. Although the thymus may involute in response to hormones or season, age involution is inconsistent, and the thymus may be found in many older fish.
Evolution of the Immune System
Immunity in Invertebrates
Innate Immunity
Prophenoloxidase (proPO)-Activating System
RNA Interference
Immunity in Cyclostomes
Immunological “Big Bang”
Immunity in Jawed Fish
Innate Immunity
Adaptive Immunity
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Evolution of the Immune System
Only gold members can continue reading. Log In or Register to continue