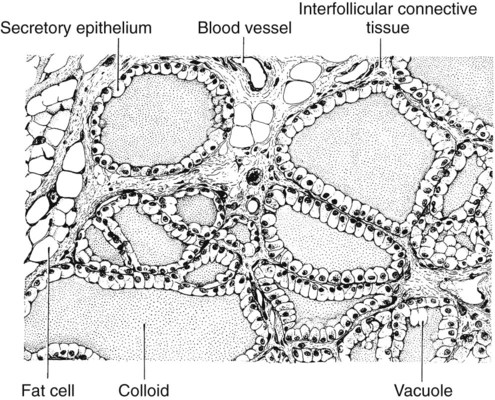
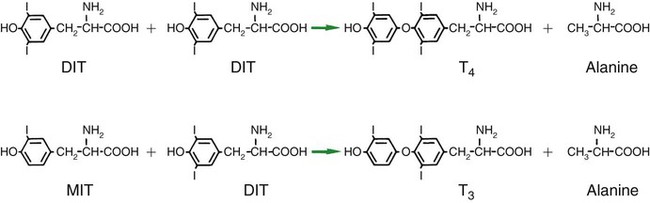
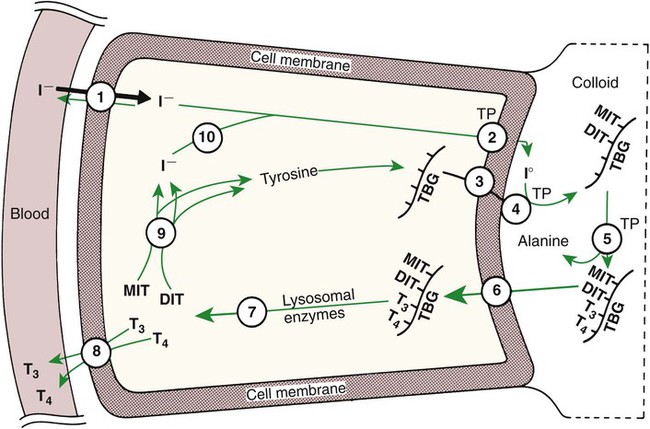
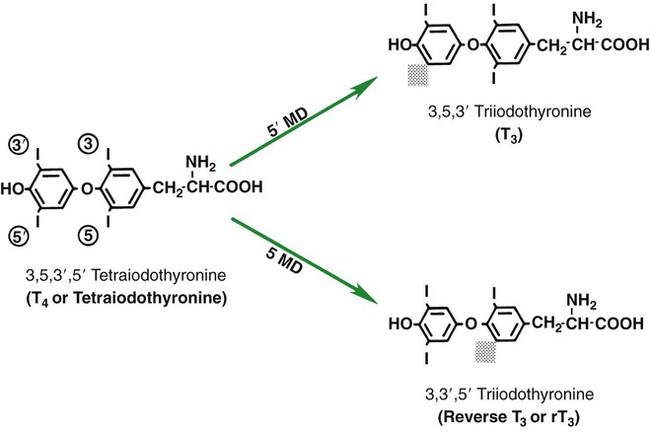
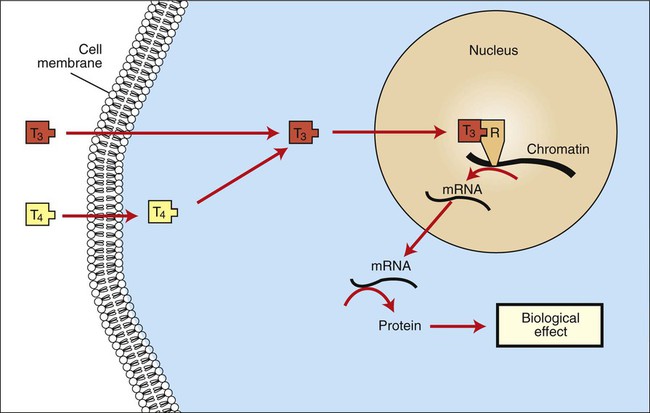
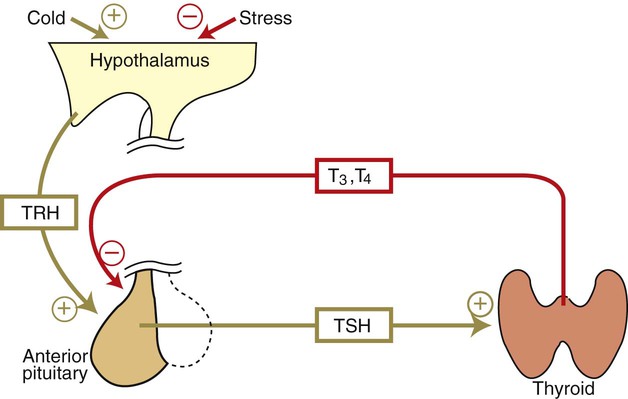
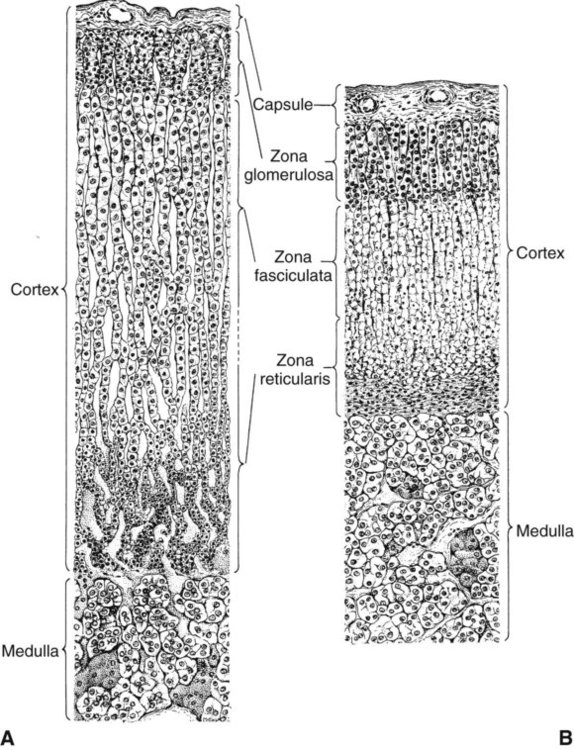
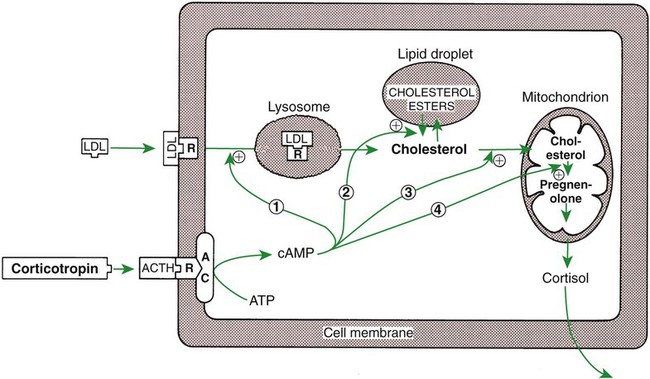
1. The thyroid hormones are synthesized from two connected tyrosine molecules that contain three or four iodine molecules. 2. Thyroid hormones are stored outside the cell and attached to thyroglobulin in the form of colloid. 3. The release of thyroid hormones involves transport of thyroglobulin with attached thyroid hormones into the cell, cleavage of the thyroid hormones from thyroxine-binding globulin, and release into the interstitial tissues. 4. Thyroid hormones are transported in the plasma attached to plasma proteins. 5. The main routes of metabolism of thyroid hormones are through deiodination or the formation of glucuronides and sulfates via hepatic mechanisms. 6. Thyroid hormones are the primary factors for the control of basal metabolism. 7. The ingestion of compounds that inhibit the uptake or organic binding of iodine blocks the thyroid’s ability to secrete thyroid hormones and causes goiter. 1. The adrenal glands are composed of two organs: the outer gland (cortex) and the inner gland (medulla). 1. The adrenal cortex has three zones: the zona glomerulosa, which secretes mineralocorticoids, and the zona fasciculata and the zona reticularis, which secrete glucocorticoids and sex steroids. 2. Adrenal corticoids are synthesized from cholesterol; the critical difference in the activity of these corticoids is related to the hydroxyl group on C-17 of glucocorticoids. 3. Adrenocortical hormones are carried in plasma in association with specific binding globulins (corticosteroid-binding globulin). 4. The metabolism of adrenocortical hormones involves the reduction of double bonds and conjugation of the steroids to glucuronides and sulfates. 5. One of the most important functions of glucocorticoids is control of metabolism, in particular the stimulation of hepatic gluconeogenesis. 6. Corticotropin is the pituitary hormone that regulates glucocorticoid synthesis by the adrenal cortex. 7. One of the most important clinical uses of glucocorticoids is the suppression of the inflammatory response. 1. The synthesis of catecholamines is from tyrosine; the main catecholamine synthesized by the adrenal medulla is epinephrine. 2. The primary actions of catecholamines are on metabolism, especially effects that increase the concentration of glucose. 3. The main factors that stimulate catecholamine secretion are hypoglycemia and conditions that produce stress. 1. The synthesis of insulin is biphasic: an acute phase involves the release of preformed insulin, and a chronic phase involves the synthesis of protein. 2. The metabolism of insulin involves splitting the A and B chains and reducing the chains to amino acids and peptides. 3. The main metabolic functions of insulin are anabolic. 4. Insulin deficiency produces diabetes mellitus, which can culminate in diabetic ketoacidosis. 5. Dietary management is an important consideration in therapy for feline type 2 diabetes. 6. The most important functions of glucagon are to decrease glycogen synthesis, increase glycogenolysis, and increase gluconeogenesis. 7. Glucagon synthesis is stimulated by decreased glucose concentrations in the blood. 8. The main functions of somatostatin are to inhibit the secretion of hormones produced by the pancreas (insulin, glucagon, pancreatic polypeptide). Calcium and phosphate metabolism 1. Calcium is important for many intracellular reactions, including muscle contraction, nerve cell activity, release of hormones through exocytosis, and activation of enzymes. 2. Phosphate is important for the structure of bone and teeth, and organic phosphate serves as part of the cell membrane and several intracellular components. 3. The most important body pool of calcium involved in homeostasis is the extracellular fluid component. The thyroid gland is the most important endocrine gland for metabolic regulation. The glandular tissue has cells formed in a circular arrangement called a follicle (Figure 34-1). The follicles are filled with a homogeneous-staining substance called colloid, which is the main storage form of the thyroid hormones. The follicular cells are cuboidal when the secretion is basal and are elongated when the cells are stimulated to release hormone. Another important endocrine cell, the parafollicular cell, or C cell, is located outside the follicles. This cell secretes calcitonin, a hormone important for the regulation of calcium. The activity of this hormone is discussed in the section on calcium metabolism. As iodide passes through the apical wall of the cell, it attaches to the ring structures of the tyrosine molecules, which are part of the thyroglobulin amino acid sequence. The tyrosyl ring can accommodate two iodide molecules; if one iodide molecule attaches, it is called monoiodotyrosine, and if two attach, it is called diiodotyrosine. The coupling of two iodinated tyrosine molecules results in the formation of the main thyroid hormones; two diiodotyrosine molecules form tetraiodothyronine, or thyronine (T4), and one monoiodotyrosine and one diiodotyrosine molecule form triiodothyronine (T3) (Figure 34-2). A key enzyme in the biosynthesis of thyroid hormones is thyroperoxidase (which works in concert with an oxidant, hydrogen peroxide). Thyroperoxidase catalyzes the iodination of the tyrosyl residues of thyroxine-binding globulin (TBG) and the formation of T3 and T4. In addition to the unusual molecular storage form of the hormone, thyroid hormones are also unique in that they are the only hormones that contain a halide (i.e., iodine). For thyroid hormones to be released from the thyroid gland, thyroglobulin with its attached monoiodotyrosine, diiodotyrosine, T3, and T4 molecules must be translocated into the follicle cell, and the hormones must be cleaved from thyroglobulin (Figure 34-3). Key enzymes in this transfer are found in the lysosomes. On entering the cell, the TBG molecules fuse with lysosomes, and lysosomal enzymes cleave both the iodinated tyrosine molecules and the iodinated thyronines from the thyroglobulin molecule. The thyronines are released through the basal cell membrane (they freely pass through the cell membrane); monoiodotyrosine and diiodotyrosine are deiodinated by an enzyme called iodotyrosine dehalogenase; and both the iodide and the remaining tyrosine molecules are recycled to form new hormone in association with thyroglobulin. The majority of T3 formation occurs outside the thyroid gland by deiodination of T4. Tissues that have the highest concentration of deiodinating enzymes are those of the liver and kidneys, although muscle tissue produces more T3 on the basis of relative size. The enzyme that is involved in the removal of iodide from the outer phenolic ring of T4 in the formation of T3 is called 5′-monodeiodinase (Figure 34-4). Another type of T3 in which an iodide molecule is removed from the inner phenolic ring of T4, a compound called reverse T3, is also formed. Reverse T3 has little of the biological effects of thyroid hormones and is formed only by the action of extrathyroidal deiodinating enzymes and not by activity of the thyroid gland. As indicated in Chapter 33, lipid-soluble hormones are transported in the vascular system through association with specific binding plasma proteins. There is considerable species variation in the proteins that bind thyroid hormones. The most important carrier protein is TBG, which has high affinity for T4, although it also has low capacity because of its low concentration. TBG also is an important carrier protein for T3. TBG has been reported in all domestic animals except the cat. Albumin is also involved in the transport of thyroid hormones; however, albumin has low affinity for T3 and T4 but high capacity because of its high concentration in plasma. In the absence of TBG, albumin is the most important carrier of thyroid hormones. All species have a third plasma protein, thyroxine-binding prealbumin, which is specific for T4 and has a specificity and capacity that are intermediate between those of TBG and albumin. The term prealbumin refers to the migration of the protein during electrophoresis, not to synthesis of the molecule. The mechanism of action of thyroid hormones at the cellular level is based on their ability to penetrate the cell membrane even though they are amino acids; in essence, they are lipophilic. Although it is thought that thyroid hormones interact directly with the nucleus to initiate the transcription of messenger ribonucleic acid (mRNA) (Figure 34-5), the presence of T3 receptors has been reported on mitochondria. TSH, or thyrotropin, is the most important regulator of thyroid activity. It acts through the initiation of cyclic adenosine 3′,5′-monophosphate (cAMP) formation and the phosphorylation of protein kinases. Thyrotropin secretion is regulated by thyroid hormones through negative-feedback inhibition of the synthesis of thyrotropin-releasing hormone (TRH) at the level of the hypothalamus and by inhibition of TSH activity at the level of the pituitary gland (Figure 34-6). Diagnosis is based on measurement of serum basal total thyroxine (T4) and triiodothyronine (T3) concentrations, serum free T4 and T3 concentrations, and endogenous canine serum thyrotropin (TSH) levels (Table 34-1) and/or results of dynamic thyroid function tests, including the TRH and TSH stimulation tests. The many variables that affect T4 include age, breed, environmental and body temperature, diurnal rhythm, obesity, and malnutrition. Specifically, affected greyhounds have approximately half the normal total thyroxine (TT4) and free thyroxine (unbound) (FT4) concentrations of normal dogs. Obese dogs have mild increases in serum TT4 concentrations. In puppies the serum TT4 concentration is two to five times higher than in adult dogs. Furthermore, there is an age-related decline in serum TT4 concentrations and response to TSH stimulation in dogs. Euthyroid sick syndrome is characterized by a decrease in serum TT4 and increase in reverse T3. Concurrent illnesses such as diabetes mellitus, chronic renal failure, hepatic insufficiency, and infections can cause euthyroid sick syndrome, resulting in decreases in serum TT4 concentrations. Drugs such as anesthetics, phenobarbital, primidone, diazepam, trimethoprim-sulfa, quinidine, phenylbutazone, salicylates, and glucocorticoids can also decrease serum basal TT4 concentrations. TABLE 34-1 Serum T4 and T3 Values by Radioimmunoassay T3, Triiodothyronines; T4, thyronine; M ± SD, median plus/minus standard deviation. *N = 10, for all species listed. From McDonald LE: Veterinary endocrinology and reproduction, ed 4, Philadelphia, 1989, Lea & Febiger. More recently, investigators have honed in on the molecular aspects of feline hyperthyroidism. The disease in cats is more similar to toxic nodular goiter in humans and is characterized by autonomous growth of thyroid follicles. The pathogenesis of toxic nodular goiter is an abnormality in the signal transduction of the thyroid cell. The TSH receptor on the thyroid cells activates receptor-coupled guanosine triphosphate-binding proteins (G proteins; see Chapter 1). Uniquely, the thyroid cell proliferation and hormone production are both controlled by the TSH receptor-G-protein-cAMP signaling. Overexpression of stimulatory G proteins and underexpression of inhibitory G proteins have been demonstrated in some humans with toxic nodular goiter. Mutations of the TSH receptor that result in the receptor remaining activated without ligand (i.e., TSH) have also been reported in humans with toxic nodular goiter. The adrenal glands are two bilaterally symmetric endocrine organs located just anterior to the kidneys. Each gland is divided into two separate entities, a medulla and a cortex (Figure 34-7), each of which produces different types of hormones. These adrenal tissues have different embryonic origins. The medulla arises from the neuroectoderm and produces amines such as norepinephrine and epinephrine. The cortex arises from the mesodermal coelomic epithelium and produces steroid hormones such as cortisol, corticosterone, sex steroids, and aldosterone. The utility of placing two such disparate tissues together is not apparent. The one common factor is that both sets of hormones are important for adaptation to adverse environmental conditions (i.e., stress). The adrenal cortex is organized into three zones in mammals (see Figure 34-7). The outer zone, the zona glomerulosa, is relatively narrow, and its cells are organized in a whorl-type arrangement. The middle zone, the zona fasciculata, is relatively wide, and its cells are organized in columns. In the cow and sheep, the zona fasciculata is further divided into inner and outer layers. The inner zone of the adrenal cortex, the zona reticularis, which is adjacent to the adrenal medulla, is intermediate in size, and cells are more randomly organized. The synthesis of adrenal steroids involves the classic pathways for steroid biosynthesis. As indicated previously, cholesterol is the major starting material for the synthesis of steroid hormones. Cholesterol is readily available to the steroid-synthesizing cells because it is stored in large quantities in ester form within lipid droplets in these cells. One of the initial steps in steroid formation is the hydrolysis of the ester. The first step in steroid synthesis involves an enzyme that cleaves the carbon side chain from the steroid molecule, leaving a C-21 steroid known as pregnenolone. This step occurs within the mitochondrion (Figure 34-8). The synthesis of all steroid hormones, regardless of their form, utilizes pregnenolone in the synthetic pathway (see Figure 33-5). As emphasized previously, adrenocortical hormones are classified as either glucocorticoid or mineralocorticoid in their activity. Before the biological actions of each class are discussed, it is important to realize that there is overlap of activity (Table 34-2). For example, whereas cortisol is the dominant glucocorticoid hormone, it also has mineralocorticoid effects, although at a reduced potency. TABLE 34-2 Relative Glucocorticoid and Mineralocorticoid Potencies of Various Steroids From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders. Glucocorticoids play a role in water diuresis (i.e., the enhancement of water excretion). Whereas glucocorticoids inhibit vasopressin activity in the distal tubule, the most important effect is to increase the GFR. Table 34-3 summarizes the effects of glucocorticoids. TABLE 34-3 Glucocorticoid Effects and Target Tissues From Hedge GA, Colby HD, Goodman RL: Clinical endocrine physiology, Philadelphia, 1987, Saunders. The control of the secretion of the glucocorticoids by the zona fasciculata and zona reticularis is by the tropic hormone (corticotropin) (Figure 34-9). A negative-feedback system exists, whereby glucocorticoids inhibit the release of hypothalamic corticotropin–releasing hormone, which in turn results in decreased corticotropin secretion by the pituitary gland. Some evidence indicates that glucocorticoids also have a negative-feedback effect at the level of the pituitary gland. The potency of a glucocorticoid in negative-feedback inhibition of corticotropin is directly related to its glucocorticoid potency; for example, cortisol has more potent negative-feedback effects than corticosterone and has more potent glucocorticoid effects.
Endocrine Glands and Their Function
The Thyroid Gland
The Thyroid Hormones Are Synthesized from Two Connected Tyrosine Molecules That Contain Three or Four Iodine Molecules
The Release of Thyroid Hormones Involves Transport of Thyroglobulin with Attached Thyroid Hormones into the Cell, Cleavage of the Thyroid Hormones from Thyroxine-Binding Globulin, and Release into the Interstitial Tissues
Thyroid Hormones Are Transported in the Plasma Attached to Plasma Proteins
Thyroid Hormones Are the Primary Factors for the Control of Basal Metabolism
The Ingestion of Compounds That Inhibit the Uptake or Organic Binding of Iodine Blocks the Thyroid’s Ability to Secrete Thyroid Hormones and Causes Goiter
Hypothyroidism in Dogs
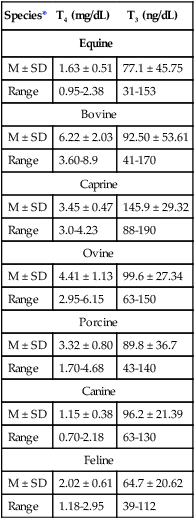
Species*
T4 (mg/dL)
T3 (ng/dL)
Equine
M ± SD
1.63 ± 0.51
77.1 ± 45.75
Range
0.95-2.38
31-153
Bovine
M ± SD
6.22 ± 2.03
92.50 ± 53.61
Range
3.60-8.9
41-170
Caprine
M ± SD
3.45 ± 0.47
145.9 ± 29.32
Range
3.0-4.23
88-190
Ovine
M ± SD
4.41 ± 1.13
99.6 ± 27.34
Range
2.95-6.15
63-150
Porcine
M ± SD
3.32 ± 0.80
89.8 ± 36.7
Range
1.70-4.68
43-140
Canine
M ± SD
1.15 ± 0.38
96.2 ± 21.39
Range
0.70-2.18
63-130
Feline
M ± SD
2.02 ± 0.61
64.7 ± 20.62
Range
1.18-2.95
39-112
Hyperthyroidism in Cats
The Adrenal Glands
The Adrenal Glands Are Composed of Two Organs: the Outer Gland (Cortex) and the Inner Gland (Medulla)
The Adrenal Cortex
The Adrenal Cortex Has Three Zones: the Zona Glomerulosa, Which Secretes Mineralocorticoids, and the Zona Fasciculata and the Zona Reticularis, Which Secrete Glucocorticoids and Sex Steroids
Adrenal Corticoids Are Synthesized from Cholesterol; the Critical Difference in the Activity of These Corticoids Is Related to the Hydroxyl Group on C-17 of Glucocorticoids
One of the Most Important Functions of Glucocorticoids Is Control of Metabolism, in Particular the Stimulation of Hepatic Gluconeogenesis
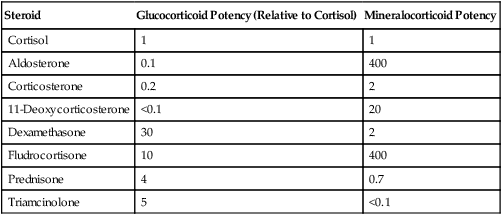
Steroid
Glucocorticoid Potency (Relative to Cortisol)
Mineralocorticoid Potency
Cortisol
1
1
Aldosterone
0.1
400
Corticosterone
0.2
2
11-Deoxycorticosterone
<0.1
20
Dexamethasone
30
2
Fludrocortisone
10
400
Prednisone
4
0.7
Triamcinolone
5
<0.1
Effect
Site of Action
Stimulates gluconeogenesis
Liver
Increases hepatic glycogen
Liver
Increases blood glucose
Liver
Facilitates lipolysis
Adipose tissue
Is catabolic (negative nitrogen balance)
Muscle, liver
Inhibits corticotropin secretion
Hypothalamus, anterior pituitary gland
Facilitates water excretion
Kidney
Blocks inflammatory response
Multiple sites
Suppresses immune system
Macrophages, lymphocytes
Stimulates gastric acid secretion
Stomach
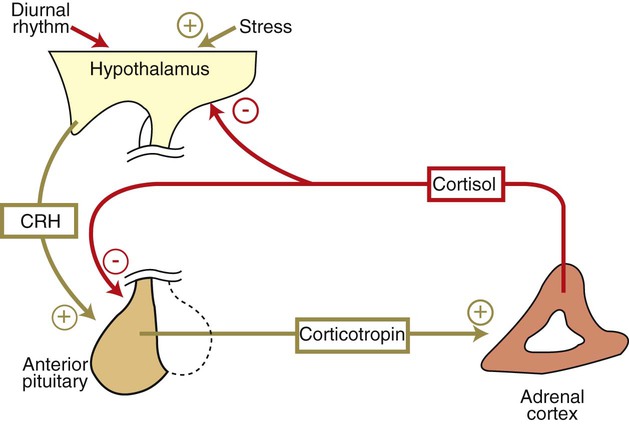
Corticotropin Is the Pituitary Hormone that Regulates Glucocorticoid Synthesis by the Adrenal Cortex
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Endocrine Glands and Their Function
Only gold members can continue reading. Log In or Register to continue