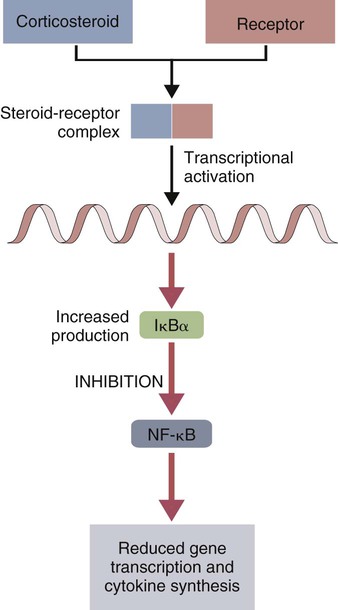
• There are many drugs that can suppress immune responses. The most widely used are corticosteroids. These prevent the activation of NF-κB and block many immune functions. • Immunosuppressive drugs used to prevent allograft rejection or to treat autoimmune disease may either be nonspecific inhibitors of cell division or specifically block the activation of T cells by interfering with signal transduction. • Drugs employed to stimulate the immune system commonly include microbial molecules that activate toll-like receptors (TLRs) in a nonspecific fashion. • Cytokine therapy may eventually be useful in veterinary medicine, but the toxicity and cost of these molecules have so far prevented widespread clinical use. The effects of corticosteroids on cell function have a common pathway (Figure 39-1). Corticosteroids are absorbed directly into cells, where they bind to receptors in the cytosol. The corticosteroid-receptor complexes are then transported to the nucleus, where they stimulate the synthesis of IκBα, the inhibitor of NF-κB. In a resting cell, NF-κB is inactive since its nuclear binding site is masked by IκBα. When a lymphocyte is stimulated, the two molecules dissociate, the IκBα is degraded by proteasomes, and the released NF-κB moves to the nucleus and activates genes involved in inflammation and immunity. Corticosteroids, however, stimulate the production of excess IκBα. This excess continues to block NF-κB-mediated processes, including cytokine synthesis and T cell responses. As a result, corticosteroids suppress both immunological and inflammatory processes. Corticosteroids influence immunity in four areas (Box 39-1): they affect leukocyte production and circulation; they influence the effector mechanisms of lymphocytes; they modulate the activities of inflammatory mediators; and they modify protein, carbohydrate, and fat metabolism. Corticosteroids cause apoptosis of thymocytes and thus induce thymic atrophy. They also suppress the ability of T cells to produce cytokines. The most important exception to this is IL-2, which is not regulated by NF-κB (Chapter 8). Lymphocyte proliferation in response to foreign cells is suppressed, suggesting that there is interference with the recognition of MHC class II molecules. Corticosteroids also block production of lymphotoxin. NK and some antibody-dependent cellular cytotoxicity (ADCC) reactions may be refractory to corticosteroid treatment, although in cattle corticosteroids may increase serum interferon (IFN) levels. The effects of corticosteroids on antibody responses are variable and depend on timing and dose. In general, B cells tend to be corticosteroid resistant, and enormous doses are usually required to suppress antibody synthesis. It is interesting to note, however, that in horses, moderate doses of dexamethasone suppress IgG1 and IgG4 responses while having no apparent effect on IgG3 responses. Corticosteroids also upregulate the expression of CD121b. This is a decoy receptor that can bind active IL-1 but will not transduce a signal, effectively blocking IL-1 activity. Alkylating agents cross-link DNA helices, preventing their separation, and thus block cell division. The most important of these is cyclophosphamide (Figure 39-2). Cyclophosphamide is toxic for resting and dividing cells, especially for dividing immunocompetent cells. It impairs both B and T cell responses, especially the primary immune response. It blocks mitogen and antigen-induced cell division and the production of IFN-γ. It prevents B cells from renewing their antigen receptors. Early in therapy, cyclophosphamide tends to destroy more B cells than T cells. In long-term therapy it affects both cell populations. It also suppresses macrophage function. Cyclophosphamide may be administered parenterally or orally and is inactive until biotransformed in the liver. It has a half-life of about 6 hours and is largely excreted through the kidney. It is of interest to note that corticosteroids enhance the metabolism of cyclophosphamide and so reduce its potency. The main toxic effect of cyclophosphamide is bone marrow suppression, leading to leukopenia with a predisposition to infection. Other adverse effects may include thrombocytopenia, anemia, and bladder damage. Cyclophosphamide may be of benefit in the treatment of lymphoid neoplasia and in the treatment of immune-mediated skin diseases, although its potential toxicity suggests that other, less-toxic alternatives be considered first.
Drugs and Other Agents That Affect the Immune System
Nonspecific Immunosuppression
Radiation
Corticosteroids
Cytotoxic Drugs
Alkylating Agents
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drugs and Other Agents That Affect the Immune System
Only gold members can continue reading. Log In or Register to continue