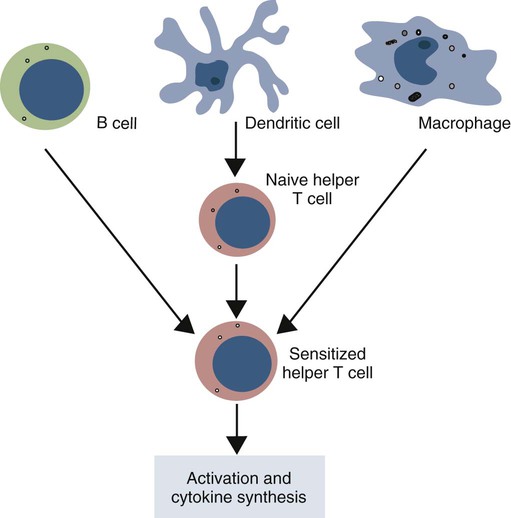
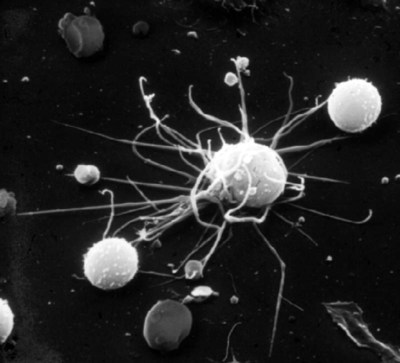
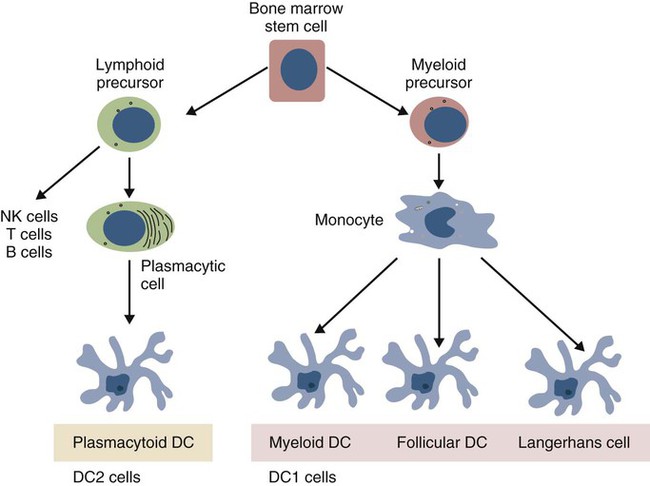
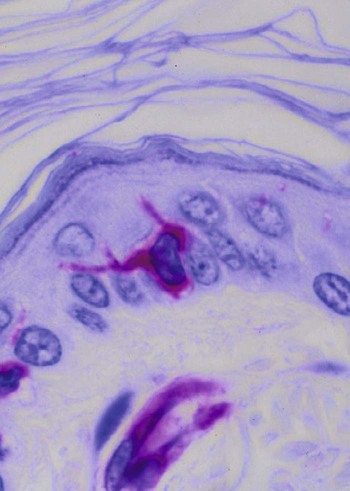
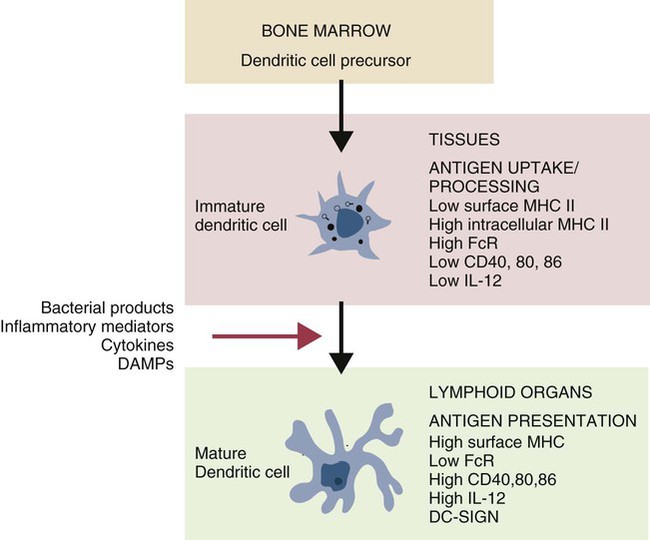
• Dendritic cells, macrophages, and B cells can capture and process foreign antigens so that they will trigger adaptive immune responses. • Dendritic cells are the most efficient of these antigen-processing cells. Only dendritic cells can effectively stimulate naïve T cells. • Immature dendritic cells are found throughout the body. They are especially equipped to capture and process antigens. • Once stimulated by antigens, dendritic cells mature and become highly effective in presenting these processed antigens to T cells. They express high levels of antigen receptors called major histocompatibility complex (MHC) class II molecules on their surface. • Dendritic cells ingest antigens, break them into small fragments, and then present them on their surface MHC molecules, where they can be recognized by T cells. • Macrophages also act as antigen-presenting cells, but since they also destroy ingested antigens, they are much less efficient than dendritic cells. • B cells can act as antigen-presenting cells and are especially effective during secondary immune responses. The organisms that trigger adaptive immune responses are of two general types. One type is typified by the bacteria that invade the body from outside and then grow in the tissues and extracellular fluid. Their antigens are called exogenous antigens, and they are processed by specialized antigen-processing cells. A second type of invading organism is typified by viruses that invade a cell and force it to make viral proteins. These new proteins are called endogenous antigens. Endogenous antigens are processed by the cells in which they are produced. There are two classes of MHC molecules called MHC class I and MHC class II. MHC class I molecules are made by all nucleated cells and bind endogenous antigens. MHC class II molecules, in contrast, are restricted to specialized antigen-processing cells and bind exogenous antigens. The body mainly employs three cell types: dendritic cells, macrophages, and B cells to process exogenous antigens. The most important of these are dendritic cells (Figure 10-1). The shape of dendritic cells depends on their state of activation. Typically, however, they are characterized by having a small cell body with many long cytoplasmic processes known as dendrites (Figure 10-2). The dendrites increase the efficiency of antigen trapping and maximize contact between dendritic cells and other cells. Dendritic cells are a mixture of several different subpopulations. Thus they are divided into myeloid (M-DC) and plasmacytoid (P-DC) dendritic cells (Figure 10-3). These two subpopulations differ in morphology, in surface antigens, and in their functions, although they share adhesion molecules, co-stimulatory molecules, and activation markers. Other specialized dendritic cell subpopulations are found in the skin (Langerhans cells) and in lymphoid organs (follicular dendritic cells). As pointed out earlier, the adaptive immune system has two major branches: the antibody-mediated and cell-mediated immune responses. The type of immune response mounted by an animal is determined by the type of helper T (Th) cells triggered in response to an antigen. Thus there are several types of Th cells (see Figure 14-2). One major type, Th1 cells, stimulates cell-mediated immune responses designed to protect animals against intracellular organisms. The other major type, Th2 cells, stimulates antibody-mediated immune responses designed to protect animals against extracellular invaders. Which Th cell type is activated depends on the use of different dendritic cell subpopulations. Blood monocytes are the immediate precursors of both tissue macrophages and M-DCs. Which of these cell types is produced depends on the mixture of cytokines and cells encountered by the monocyte as it differentiates. Each cell type can convert to the other until late in the differentiation process. M-DCs can therefore be considered part of the mononuclear phagocytic system being derived from a common stem cell, respond to the same growth factors, express the same surface markers, and in effect are in no specific way uniquely different from other macrophages. Thus macrophages may consist of a spectrum of cell types ranging from highly effective antigen presenters (dendritic cells) at one extreme to suppressors of T cell activation (M2 cells) at the other. Monocytes exposed to certain T cell cytokines differentiate into M-DCs, and functionally different dendritic cells can be induced according to the local cytokine environment. Bovine peripheral blood monocytes exposed to staphylococcal enterotoxin C1, a superantigen (Chapter 14), will convert to dendritic cells. P-DCs are long-lived cells found in blood, bone marrow, and lymphoid organs. They are specialized to respond to viruses by producing massive amounts of the type I interferons (IFN-α and IFN-β). Their numbers increase during infection. It is possible that the P-DCs serve as an early warning system for viral infections since they are rapidly activated by viral nucleic acids. Plasmacytoid dendritic cells have a unique ability to link innate and adaptive immunity. After producing large amounts of type I interferon, they are still able to differentiate into mature DCs that can stimulate naïve T cells. Because P-DCs secrete large amounts of IFN-α, they also activate natural killer (NK) cells (Chapter 19). Several dendritic cell subpopulations are found in skin. Langerhans cells, for example, are specialized, long-lived M-DCs found in the epidermis. Their long dendrites form an extensive network that is ideally situated to capture antigens (Figure 10-4). These antigens include not only invading microbes but also topically applied antigens, such as the resins of poison ivy, or intradermally injected antigens, such as those in mosquito saliva. Langerhans cells express multiple pattern-recognition receptors (PRRs), including the C-type lectins langerin and DC-SIGN that can bind bacteria, fungi, and some viruses. Langerhans cells influence the development of skin immune responses, such as delayed hypersensitivity and allergic contact dermatitis (Chapter 31). Langerhans cells contain characteristic rod- or racquet-shaped cytoplasmic granules called Birbeck granules whose function is unclear. Once antigens are captured, the Langerhans cells migrate to draining lymph nodes, where they present the antigen to T cells. Specialized dendritic cells called follicular dendritic cells are found in secondary lymphoid organs (Chapter 12). They are a form of M-DC derived from stromal cell precursors. Follicular dendritic cells present antigens to B cells in two different ways. In an animal that has not previously been exposed to the antigen, antigen presentation is a passive process. The dendritic cells simply provide a surface on which antigen can be presented. In contrast, in animals that have previously been exposed to an antigen and possess antibodies, the antigen and antibody combine to form antibody-antigen complexes (also called immune complexes). Follicular dendritic cells take up these immune complexes on their surface and then shed them in membrane vesicles called exosomes. B cells can take up these exosomes and after processing the antigen present it to antigen-sensitive T cells. Follicular dendritic cells can retain antigens on their surface for more than 3 months. They integrate signals from toll-like receptors (TLRs) and other sources to support effective germinal center responses. Follicular dendritic cells play an important role in promoting immunoglobulin A (IgA) responses in Peyer’s patches in the intestinal wall (Chapter 22). They respond to lipopolysaccharides, lipopeptides, and retinoic acid from the gut flora and support the recruitment and survival of IgA-producing B cells. Although many subpopulations of dendritic cells have been characterized, their most important division is based on their state of maturity (Figure 10-5). Thus immature dendritic cells are highly specialized and efficient antigen-trapping cells. As they mature, dendritic cells undergo cellular reorganization and become specialized and efficient antigen-presenting cells.
Dendritic Cells and Antigen Processing
Dendritic Cells
Structure
Subpopulations
Myeloid Dendritic Cells
Plasmacytoid Dendritic Cells
Langerhans Cells
Follicular Dendritic Cells
Dendritic Cell Maturation
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Dendritic Cells and Antigen Processing
Only gold members can continue reading. Log In or Register to continue