Clinical signs
Sample to submit for testing
Hepatitis
Liver
Drop in egg production
Feces/large intestines, vaginal swabs
Lameness
Synovial fluid, tendons, heart, liver
Respiratory
Tracheas, tracheal swabs, feces, cloacal swabs, vaginal swabs (if also present with decreased egg production)
Dyspnea
Tracheal swabs, tracheas, eyelids, lungs
Nephritis, swollen kidneys, flushing
Kidneys
Tumors
Heparinized whole blood, tumor, plasma
Immunosuppression
Bursa, thymus, spleen, bone marrow
Skin lesions
Scab
Fowlpox
Pock lesion for cutaneous disease or trachea if diphtheritic disease
Neurological signs
Brains
Enteritis, malabsorption
Intestines, duodenal loop, feces
Proventriculitis
Proventriculus
Swabs, fluids, flushes, feces, and blood are convenient and appropriate samples to collect from live animals as are biopsies from affected sites. Fresh tissues or tissue scrapings are normally obtained from dead animals at necropsy. Unfixed tissue samples are the best specimens for pathogen detection. Nevertheless, most laboratories find them difficult to deal with due to the need for processing before nucleic acid extraction. This tissue processing is messy and must be performed consistently for repeatable and comparable results. Fixed tissues are sometimes the only sample available for analysis. The type of fixative and the time elapsed from fixation make these samples very unreliable, although useful for pathogen molecular detection if no other samples are available [11].
The method of choice for each laboratory must be based on the diagnostic needs, cost/benefit, and workload.
Nucleic acid-based molecular tests are some of the most versatile, as they are capable of detecting the pathogen directly in the sample and can be performed on a variety of sample types. Of course, the quality and quantity of the sample are of paramount importance for optimal results. Samples for PCR should be obtained with care to avoid contamination from other sites, the environment, other animals, etc., and shipped to the testing laboratory as soon as possible in sterile individual containers. The samples should be kept cold during shipping and moist with sterile saline. After their arrival to the laboratory, fluids, swabs, and flushes do not require processing prior to NA extraction and can be added directly to the lysis buffer of the nucleic acid extraction kit at the volume recommended by the manufacturer. Occasionally, these samples may be enriched or diluted in selective or enrichment media before processing, but in most cases this is not necessary. Tissues, however, always require processing prior to nucleic acid extraction.
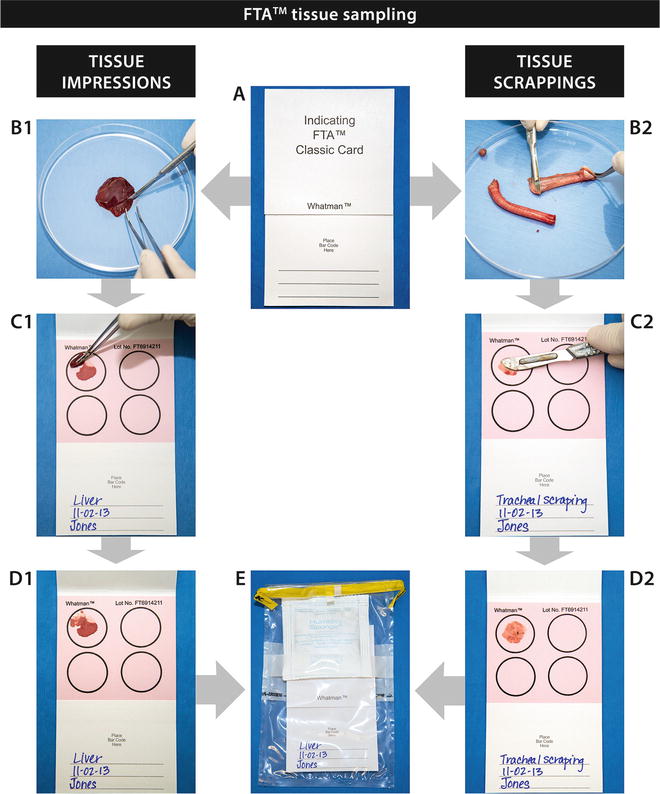
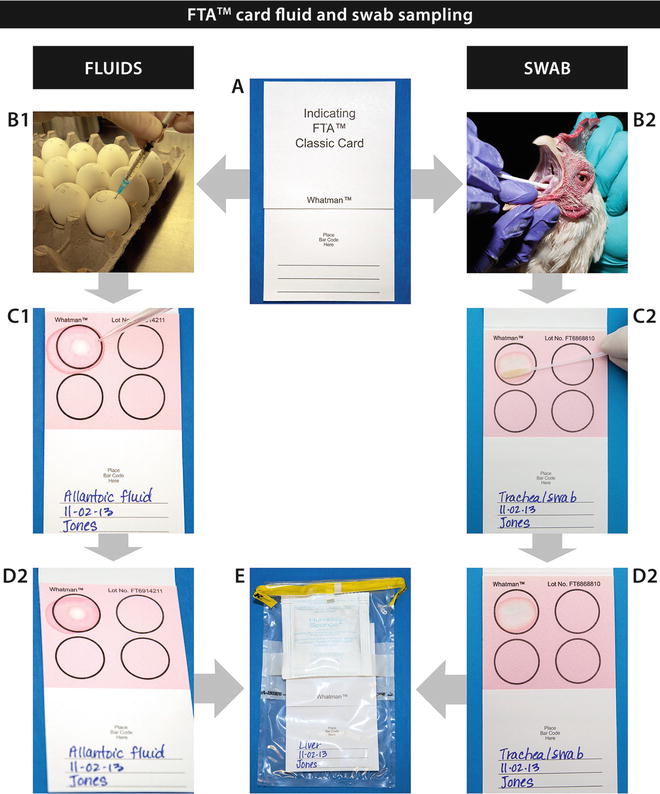
If transport to the laboratory must be delayed, samples can be placed in RNA Later solution (there are many different suppliers of this product such as Ambion Inc., Austin, TX; Life Technologies, Grand Island, NY; Qiagen, Hilden, Germany) or DNA/RNA Shield (Zymo Research, Irvine, CA). The solutions containing nucleic acid preservatives inactivate viruses and nucleases and allow the storage of samples for up to a month at room temperature. Samples can also be refrigerated overnight or frozen at −20 or −80 °C for longer holding periods, until ready for shipment. The samples should be shipped frozen with enough ice to ensure that they arrive frozen to the testing laboratory. It is not always possible to maintain the cold chain; however, the use of FTA™ cards (Whatman, GE Healthcare, Piscataway, NJ) is a viable alternative for sample preservation. One drawback of this method is that the samples are then only useful for NA detection, but not culture, cytology, direct antigen detection, etc. [12–14].
The use of FTA™ cards has greatly facilitated the collection, handling, and shipment of samples for nucleic acid testing worldwide. FTA™ is an acronym for Flinders Technology Associates and the cards were originally developed in the 1980s by Burgoyne and Fowl at Flinders University, in Australia, as a method for protecting nucleic acid samples from degradation by nucleases and other processes. The patented FTA™ cards contain proprietary chemicals, integrated within a filter paper matrix, that lyse cells, denature proteins, and immobilize and preserve nucleic acids from just about any sample type. Samples can be stored and shipped at room temperature without special handling. The integrity of the samples is optimized when cards are stored in an airtight pouch with a desiccant. The cards are available in several formats including the FTA™ Indicator Card with a color indicator that changes from pink to white following sample application, making it ideal for application of colorless samples. Nucleic acids can be purified from just a couple of punches excised from the FTA™ card. We will describe later how to apply fluids and tissues on FTA™ cards for preservation of NA and shipping to the laboratory.
In this chapter, we will describe several methods to prepare tissues in a standardized manner, as well as describe the application of several sample types to FTA™ cards. Finally, the chapter will address FTA™ card processing for nucleic acid amplification. The Figs. 1, 2, 3, and 4 included for these processes are intended to be self-explanatory and easy to follow and give the operator well-defined visuals for sample processing. The figures are the result of many years of experience in busy laboratories with a heavy caseload.
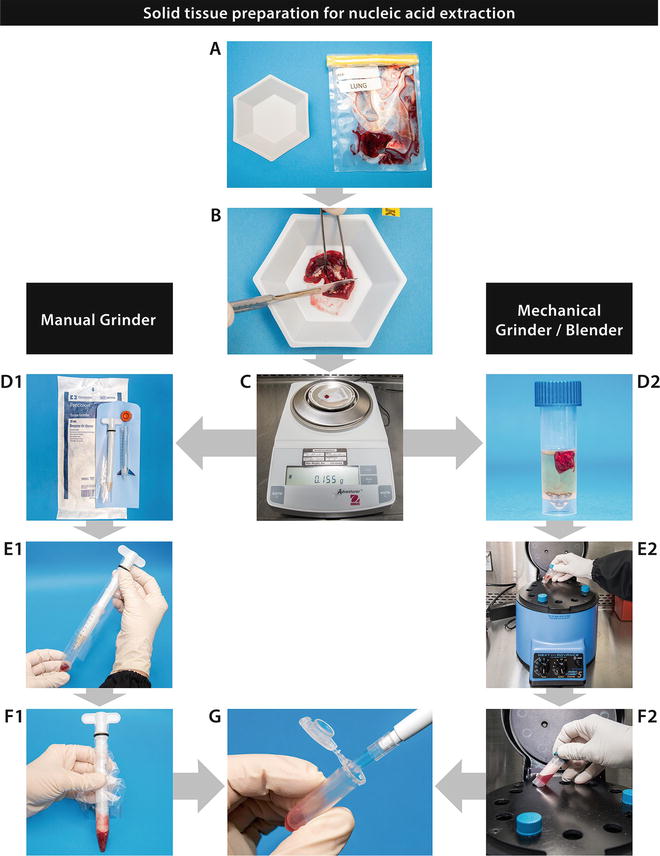
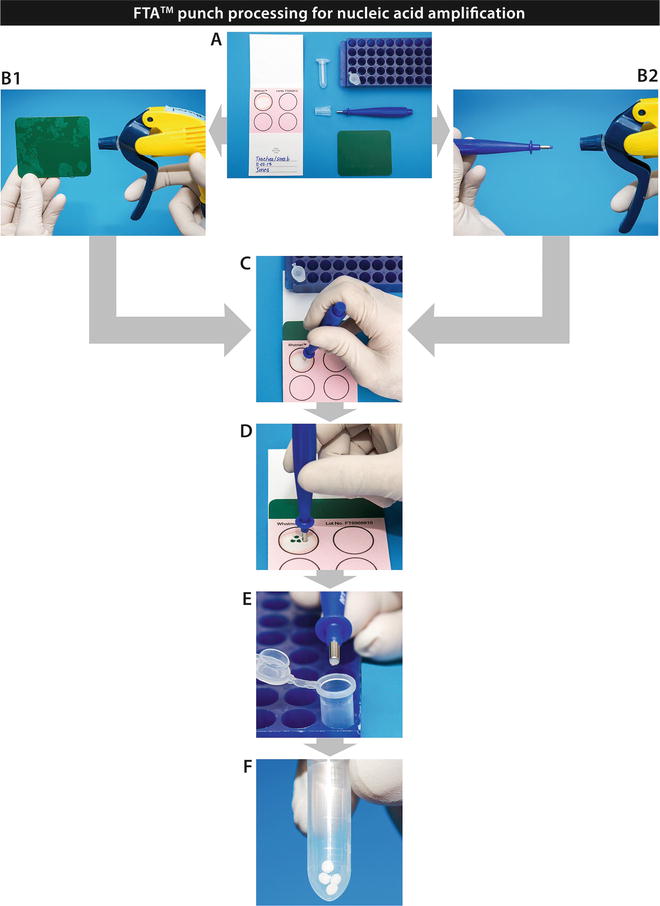

Fig. 1
Solid tissue preparation for nucleic acid extraction (see Subheading 3 for further information)

Fig. 4
FTA™ punch processing for nucleic acid amplification (see Subheading 3 for further information)
2 Materials
2.1 Fresh Tissue Processing
2.1.1 Manual and Mechanical Grinder Common Materials Needed
1.
Brain heart infusion broth (BHI).
2.
Disposable weigh dish.
3.
Scalpel and scalpel blades.
4.
Sterile forceps.
5.
Sterile pipette tips.
6.
Pipette able to dispense 2 ml, 3 ml, and 200 μl.
7.
Gloves.
8.
Laboratory dedicated wear.
2.1.2 Manual Grinder Specific Material Needed
1.
Small manual tissue grinder. Many manufacturers provide disposable grinders in sterile packages. Nevertheless, pestle and mortar or other similar manual instruments can be used. The advantage of the disposable is the fact that they have a plastic sleeve that will protect the user from splashes enhancing safety as well as minimize sample to sample contamination. See Fig. 1b for an example of a disposable manual grinder.
2.1.3 Mechanical Grinder Specific Material Needed
1.
Mechanical grinder. There are several mechanical grinders from different manufacturers. Some examples are Bullet Blender (Next Advance Averill Park, USA), TissueLyser (Qiagen), and Tissue Homogenizer (Precellys, Bertin Technologies). In our laboratory we use Bullet Blender preferentially. The protocol described here is for using this specific equipment.
2.
Stainless steel beads 0.35 mm. Optimal for all tissue types of 0.150–0.200 g. Other bead sizes may be necessary based on the size and type of tissue that will be processed. The manufacturer of the blender will likely carry beads of many different sizes, made of many materials in their catalog, available for purchase. Decide which ones are appropriate for your application and laboratory.
3.
5 ml sturdy tubes.
2.2 Materials List for Sample Collection for FTA™ Cards
2.
Whirl pack bag or similar air tight storage pouch.
3.
Desiccant packet.
4.
Pipette (50–250 μl).
5.
Sterile, RNAse-/DNAse-free pipette tips.
6.
Dacron, polyester, or flocked sterile swabs.
7.
Sterile scalpel.
8.
Sterile scissors and forceps.
9.
Sterile petri dish.
10.
Gloves.
2.3 Materials List for FTA™ Card Punch Collection (Fig. 4a)
1.
Harris Uni-Core punch (or similar).
2.
Cutting mat.
3.
70 % Ethanol.
4.
Sterile forceps.
5.
RNAse-/DNAse-free microcentrifuge tube.
6.
Gloves.
3 Methods
3.1 Tissues Preparation (Fig. 1; See Notes 1 , 2 , and 5 )
3.1.1 Manual Grinder
1.




Label a sterile bead tube with the sample identification number.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree