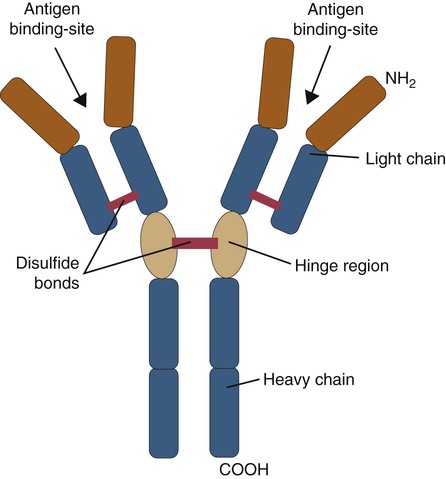
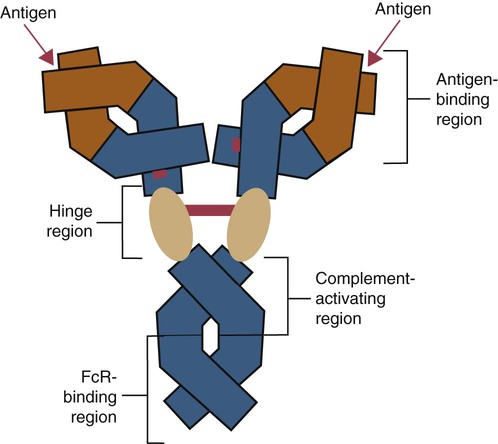
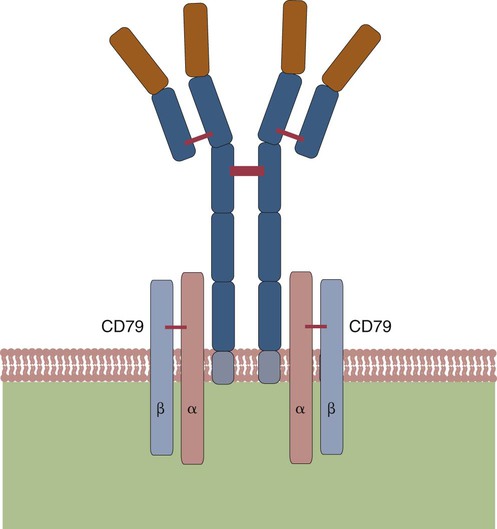
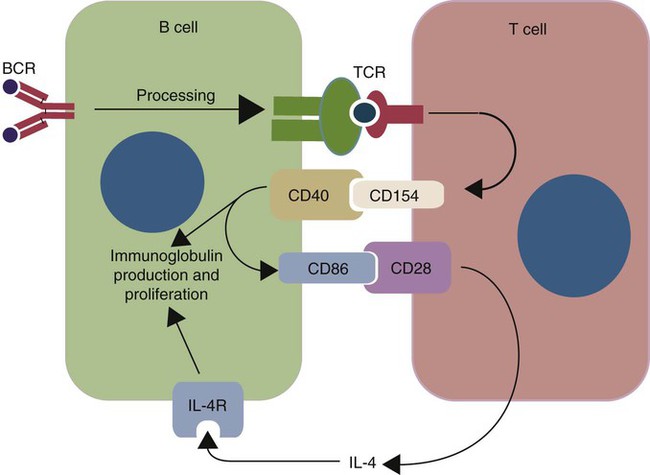
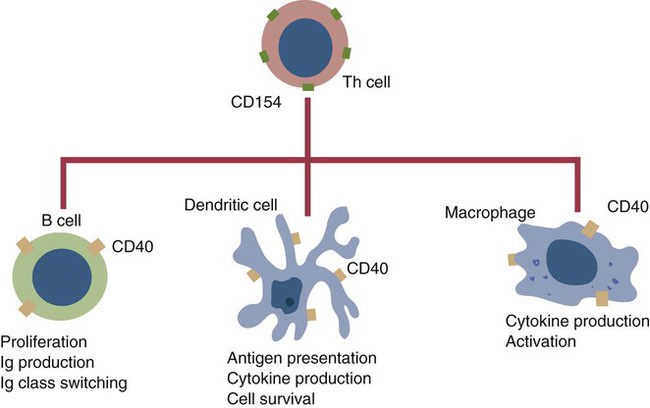
• B cells express multiple identical antigen binding receptors (BCRs) on their surface. • When BCRs are shed into body fluids, they are called immunoglobulins or antibodies. • BCRs consist of two heavy and two light chains bound together by disulfide bonds. • B cells can recognize most antigens without prior processing. However, an optimal B cell response normally requires additional stimulation by helper T cells. • Helper T cells stimulate B cells through an immunologic synapse containing costimulatory molecules and interactive receptors. • B cells require co-stimulation by cytokines. • Responding B cells may become either memory cells or antibody-secreting plasma cells. • Plasma cells are the progeny of B cells that have differentiated to secrete very large amounts of antibodies. • The differentiation of B cells into plasma cells takes place in the germinal centers of lymph nodes and other secondary lymphoid organs. • Cancerous plasma cells, called myeloma cells, produce large quantities of very pure immunoglobulin. If fused with a normal plasma cell, the resulting hybridomas can produce large quantities of pure monoclonal antibodies. B cells are mainly found in the cortex of lymph nodes, in the marginal zone in the spleen, in the bone marrow, throughout the intestine and in Peyer’s patches. Few B cells circulate in the blood. Like T cells, B cells have a large number of identical antigen-binding receptors on their surface. Each B cell, therefore, can only bind and respond to a single antigen. Antigen receptors are generated at random during B cell development in a process described in Chapter 17. If a B cell encounters an antigen that can bind its receptors, it will, with appropriate co-stimulation, respond by secreting its receptors into body fluids, where they are called antibodies. Each B cell thus makes antibodies of the same binding specificity as its receptors. Each B cell is covered with about 200,000 to 500,000 identical antigen receptors (BCRs), many more than the 30,000 antigen receptors (TCRs) expressed on each T cell. Each BCR is constructed from multiple peptide chains and, like the TCR, can be divided into antigen-binding and signaling components. Unlike the TCR, however, the BCR can also bind antigens when in solution. Antibodies are simply soluble BCRs secreted into body fluids; they all belong to the family of proteins called immunoglobulins (Chapter 16). The antigen-binding component of the BCR (or immunoglobulin) is a glycoprotein of 160 to 180 kDa consisting of four linked peptide chains. These chains consist of two identical pairs—a pair of heavy chains, each 60 kDa in size, and a pair of light chains, about 25 kDa each (Figure 15-1). The light chains are linked by disulfide bonds to the heavy chains so that the complete molecule is shaped like the letter Y. The tail of the Y (called the Fc region) is formed from paired heavy chains and is attached to the B cell surface. The arms of the Y (called the Fab regions) are formed by paired light and heavy chains, and they bind antigens (Figure 15-2). The antigen-binding sites are formed by the grooves between the light and heavy chains. Thus each BCR has two identical antigen-binding sites. When the amino acid sequences of V domains from light and heavy chains are examined in detail, two features become apparent. First, their sequence variation is largely confined to three regions, each consisting of 6 to 10 amino acids, within the variable domain (Figure 15-3). These regions are said to be hypervariable. Between the three hypervariable regions are relatively constant sequences called framework regions. The hypervariable regions on paired light and heavy chains determine the shape of the antigen-binding site and thus the specificity of antigen binding. Since the shape of the antibody-binding site is complementary to the conformation of the antigenic determinant, the hypervariable sequences are also called complementarity determining regions (CDRs). Each V-domain is folded in such a way that its three CDRs come into close contact with the antigen (Figure 15-4). Since heavy chains are paired, the domains in each chain come together to form structures by which antibody molecules can exert their biological functions. Thus VH and VL together form an antigen-binding site, and CH1 and CL together stabilize the antigen-binding site. The paired CH2 domains of IgG contain a site that activates the classical pathway of the complement system (Chapter 7) and a site that binds to Fc receptors on phagocytic cells (Figure 15-5). The heavy chain also regulates the transfer of IgG into colostrum (Chapter 21) and antibody-mediated cellular cytotoxicity (Chapter 18). When immunoglobulin molecules act as BCRs, part of their Fc region is embedded in the B cell surface membrane. These cell-bound immunoglobulins differ from the secreted form in that they have a small transmembrane domain located at their C-terminus. This contains hydrophobic amino acids that associate with the cell-membrane lipids. One important feature of the immunoglobulins is that the Fab regions, which contain the antigen-binding sites, can swing freely around the center of the molecule as if hinged. This hinge consists of a short domain of about 12 amino acids located between the CH1 and CH2 domains. The hinge region contains many hydrophilic and proline residues that cause the peptide chain to unfold and make this region readily accessible to proteases (Figure 16-10). This region also contains all the interchain disulfide bonds. Proline, because of its configuration, produces a 90-degree bend when inserted in a polypeptide chain. Because amino acids can rotate around peptide bonds, the effect of closely spaced proline residues is to produce a universal joint around which the immunoglobulin chains can swing freely. The µ chains of IgM do not possess a hinge region. BCR immunoglobulins cannot signal directly to their B cell since their cytoplasmic domains contain only three amino acids. However, their CH4 and transmembrane domains associate with glycoprotein heterodimers formed by pairing CD79a (Ig-α) with CD79b (Ig-β). These heterodimers act as signal transducers (Figure 15-6). The CD79b chains are identical in all BCRs. The CD79a chains differ depending on their associated heavy chains and employ different signaling pathways. BCR signaling is initiated by antigen binding. This leads to receptor clustering and subsequent phosphorylation of the immunoreceptor tyrosine-based activation motifs (ITAMs) on CD79a and CD79b. Phosphorylation of the tyrosines in these ITAMs by src family kinases activates downstream signaling cascades (see Figure 8-12). Although the binding of antigen to a BCR is an essential first step in triggering a B cell response, it is usually insufficient to trigger antibody formation. Complete activation of a B cell requires additional stimulation by helper T cells and cytokines (Figure 15-7). In order to do this, however, the helper T cells must themselves be presented with antigen. This antigen can be presented by one of the professional antigen-presenting cells, a dendritic cell, a macrophage, or even from a B cell. Thus a B cell can capture and process antigen, present it to a T cell, and then receive co-stimulation from the same T cell. B cells thus play two roles. They respond to antigen by making antibodies while at the same acting as antigen-processing cells. The helper T cells provide the B cell with co-stimulatory signals from cytokines as well as through interacting receptor pairs. B cells are effective antigen-presenting cells. Following antigen binding, the BCR may be internalized and degraded or transported to an intracellular compartment, where major histocompatibility (MHC) class II molecules and antigen fragments combine. These antigen-MHC class II complexes are carried to the B cell surface, where they are presented to helper T cells (Figure 15-8). This activates the T cells that then provide co-stimulation to the B cell and permit its full activation. Since all the antigen receptors on a single B cell are identical, each B cell can bind only one antigen. This makes them much more efficient antigen-presenting cells than macrophages that must present any foreign material that comes their way. This is especially true in a primed animal, in which large numbers of B cells can bind and present a specific antigen. As a result, B cells can activate Th cells with IL-4 is mainly produced by activated Th2 cells and mast cells. It stimulates the growth and differentiation of B cells. It also enhances their expression of MHC class II and Fc receptors. IL-4 also induces immunoglobulin class switching in B cells. For example, it stimulates IgA and IgE production (Table 15-1). The actions of IL-4 are neutralized by IFN-γ, which inhibits both IgA and IgE synthesis as well as B cell proliferation. Table 15-1 Adapted from Coffmann RL, Seymour BW, Lebman DA, et al: The role of helper T cell products in mouse B cell differentiation and isotype regulation, Immunol Rev 102:5, 1988. Cytokines alone cannot fully activate B cells. Complete activation also requires signaling through receptor pairs such as CD40 and CD154. For example, the co-stimulatory molecule CD40 is expressed on resting B cells, whereas its ligand, CD154, is expressed on activated helper T cells. CD40 must receive a signal from CD154 in order for a B cell to begin its cell cycle and upregulate its expression of IL-4 and IL-5 receptors (Figure 15-9; see Figure 15-8). The signals from CD40 synergize with those from IL-4 and IL-5 receptors to drive B cell activation, memory cell development, and immunoglobulin class switching. CD28 on T cells must also signal to CD86 on activated B cells for optimal costimulation to occur.
B Cells and Their Response to Antigen
B Cell Antigen Receptors
Antigen-Binding Component
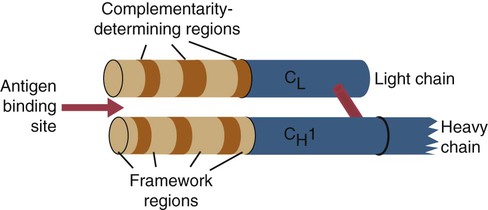
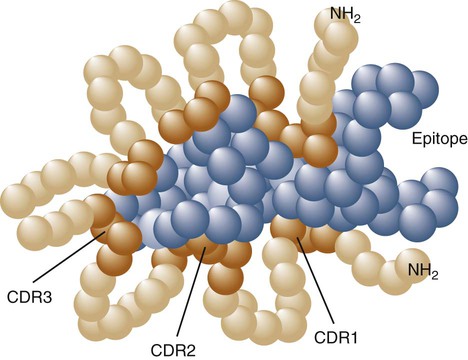
Variable Regions
Constant Regions
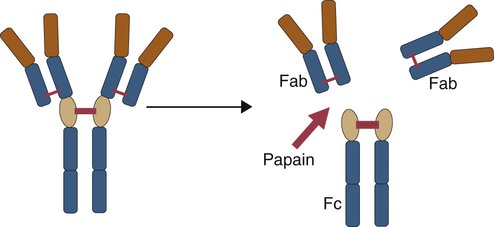
Hinge Region
Signal Transducing Component
Co-stimulation of B Cells
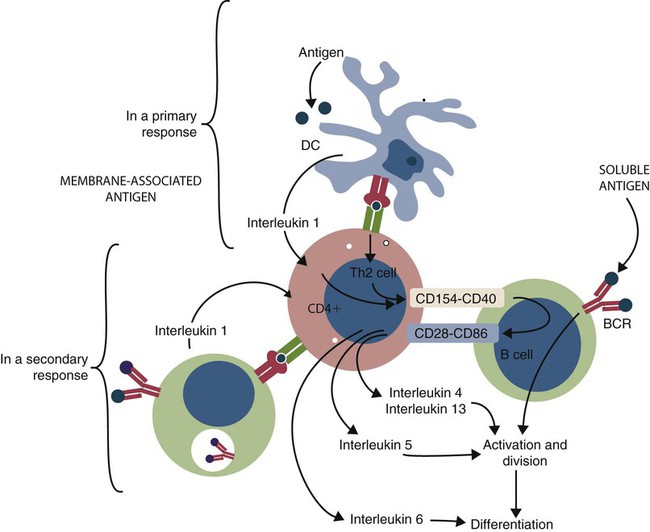
Antigen Presentation by B Cells
 of the antigen concentration required to activate macrophages.
of the antigen concentration required to activate macrophages.
Cytokine Secretion
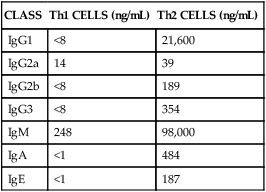
CLASS
Th1 CELLS (ng/mL)
Th2 CELLS (ng/mL)
IgG1
<8
21,600
IgG2a
14
39
IgG2b
<8
189
IgG3
<8
354
IgM
248
98,000
IgA
<1
484
IgE
<1
187
CD40 and CD154
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


B Cells and Their Response to Antigen
Only gold members can continue reading. Log In or Register to continue