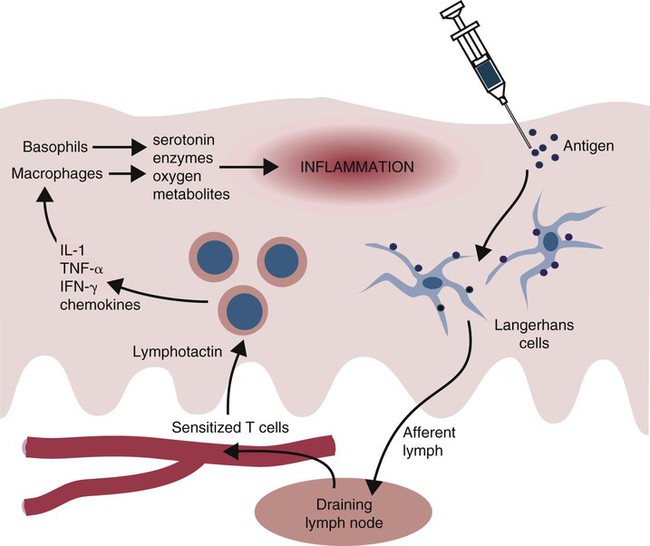
• Some antigens, when injected into the skin, induce a slowly developing inflammatory response called delayed, or type IV, hypersensitivity. • Delayed hypersensitivity reactions are mainly mediated by T cells and natural killer (NK) cells. • A good example of delayed hypersensitivity is the reaction of tuberculous cattle to intradermal injection of tuberculin. This tuberculin response provides a convenient diagnostic test for tuberculosis. • A different form of type IV hypersensitivity occurs in allergic contact dermatitis. This is a slowly developing inflammatory response that occurs when reactive chemicals bind to skin cells and trigger T cell responses. • In vitro assays for cell-mediated immunity generally focus on detecting secreted cytokines or measuring cell division induced by exposure to antigens. • In vivo assays generate a biological response such as the development of a delayed hypersensitivity skin reaction or the rejection of an allograft. When tuberculin is injected into the skin of a normal animal, there is no apparent response. On the other hand, if it is injected into an animal infected with mycobacteria, a delayed hypersensitivity response occurs. In these animals, a red, indurated (hard) swelling develops at the injection site. The inflammation begins after 12 to 24 hours, reaches its greatest intensity by 24 to 72 hours, and may persist for several weeks before fading gradually. In very severe reactions, tissue destruction and necrosis may occur at the injection site. The lesion is infiltrated with mononuclear cells (lymphocytes, macrophages), although neutrophils are present in the first hours of the reaction (Figure 31-1). When tuberculin is injected intradermally, it is taken up by Langerhans cells, which then migrate to the draining lymph node (Figure 31-2). Here they present antigen to memory T cells that respond by generating Th1 effector cells. The circulating Th1 cells recognize the antigen when they encounter it in the skin and accumulate around the antigen deposit. By 12 hours in cattle, the injection site is infiltrated with T cells. (In humans and mice, α/β T cells tend to predominate, whereas in sheep and cattle, γ/δ, WC1 T cells predominate.) There are no B cells in the lesion. Sometimes, basophils predominate in a delayed hypersensitivity reaction (Figure 31-3). This type of reaction, called cutaneous basophil hypersensitivity (CBH), can be transferred between animals with antibody, with purified B cells, or even with T cells. CBH is therefore mediated by several different mechanisms. CBH occurs in chickens in response to intradermal Rous sarcoma virus, in rabbits in response to schistosomes, and in humans with allergic contact dermatitis and renal allograft rejection. CBH reactions may contribute to the development of flea allergy dermatitis in dogs. Skin testing of cattle may be performed in several ways (Table 31-1). The simplest is the single intradermal (SID) test. In this test, 0.1 mL of PPD tuberculin derived from M. tuberculosis or Mycobacterium bovis is injected into one caudal fold (the folds of skin underneath the tail), and the injection site is examined 72 to 96 hours later. A comparison is easily made between the injected and the uninjected folds, and a positive reaction consisting of a firm lump or marked discoloration at the injection site is readily detected. Table 31-1 Tuberculin Tests Used in Cattle
Type IV Hypersensitivity
Delayed Hypersensitivity
The Tuberculin Reaction
Cutaneous Basophil Hypersensitivity
Tuberculin Reactions in Cattle
TEST
USE
ADVANTAGES
DISADVANTAGES
Single intradermal
Routine testing
Simple
Prone to false positives
Poor sensitivity
Comparative
When avian TB or Johne disease is prevalent
More specific than SID
More complex than SID
Short thermal
Use in postpartum animals and in infected animals
High efficiency
Time-consuming
Risk for anaphylaxis
Stormont
Use in postpartum animals and in advanced cases
Very sensitive and accurate
Three visits required
May sensitize an animal

< div class='tao-gold-member'>
Type IV Hypersensitivity: Delayed Hypersensitivity
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree