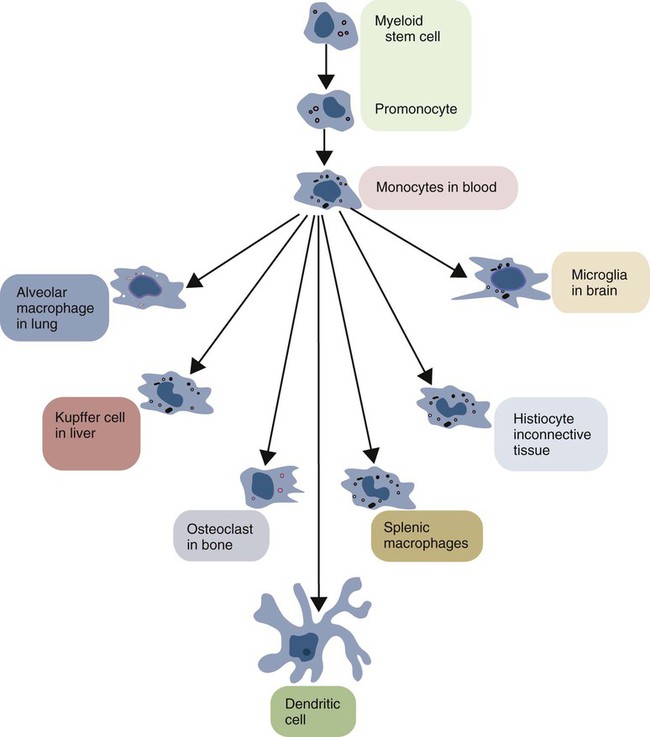
• Macrophages migrate to sites of inflammation after neutrophils. They eat and kill any surviving microbial invaders. • Macrophages also eat dead and dying neutrophils and thus prevent damage caused by escaping neutrophil enzymes. • Macrophages generate the powerful oxidizing agent, nitric oxide. • Macrophages effectively remove foreign particles from the bloodstream and the respiratory tract. • Macrophages begin the healing process in damaged tissues. • Macrophages are essential antigen-presenting cells for the adaptive immune system. Macrophage function and structure are highly variable, and this variation has given rise to a confusing nomenclature. Immature macrophages circulate in the bloodstream, where they are called monocytes. When monocytes emigrate into tissues, they become macrophages. Macrophages are found in connective tissue, where they are called histiocytes; those found lining the sinusoids of the liver are called Kupffer cells; those in the brain are microglia. The macrophages in the alveoli of the lungs are called alveolar macrophages, whereas those in the capillaries of the lung are called pulmonary intravascular macrophages. Large numbers are found in the sinusoids of the spleen, bone marrow, and lymph nodes. Irrespective of their name or location, they are all macrophages and all are part of the mononuclear phagocyte system (Figure 5-1). In suspension, monocytes are round cells about 15 nm in diameter. They possess abundant cytoplasm, at the center of which is a single large nucleus that may be round, bean shaped, or indented (Figure 5-2). Their central cytoplasm contains mitochondria, large numbers of lysosomes, some rough endoplasmic reticulum, and a Golgi apparatus, indicating that they can synthesize and secrete proteins (Figures 5-3 and 5-4). In living cells, the peripheral cytoplasm is in continuous movement, forming and reforming veil-like ruffles. Many macrophages show variations in this basic structure. For example, blood monocytes have round nuclei, which elongate as the cells mature. Alveolar macrophages contain very little rough endoplasmic reticulum, but their cytoplasm is full of granules. The microglia of the central nervous system have rod-shaped nuclei and very long cytoplasmic processes (dendrites) that are lost when the cell encounters tissue damage. Mononuclear phagocytes develop from common myeloid stem cells in the bone marrow (Figure 5-5). During monocyte development, the myeloid stem cells give rise in sequence to monoblasts, promonocytes, and eventually to monocytes, all under the influence of cytokines called colony-stimulating factors. Monocytes enter the bloodstream and circulate for about 3 days before entering tissues and developing into macrophages. They form about 5% of the total leukocyte population in blood. It is unclear whether there are subpopulations of monocytes and whether these give rise to the specialized macrophage populations. The stage at which they leave the bloodstream may determine their function. Tissue macrophages either originate directly from monocytes or arise by division within tissues. They are relatively long-lived cells, replacing themselves at a rate of about 1% per day unless activated by inflammation or tissue damage. Macrophages may live for a long time after ingesting inert particles, such as the carbon in tattoo ink, although they may fuse together to form multinucleated giant cells in their attempts to eliminate the foreign material. Myeloid stem cells may also, when appropriately stimulated, give rise to dendritic cells. Indeed, these cells are so closely related that many investigators consider that dendritic cells are simply specialized macrophages optimized for antigen processing and presentation (Chapter 10). As described in Chapter 2, macrophages express many different pattern-recognition receptors (PRRs) and readily detect and respond to invading bacteria and viruses. In addition to effective phagocytosis, they respond by producing complex cytokine mixtures. The most important of these are interleukin-1 (IL-1), IL-6, IL-12, IL-18, and tumor necrosis factor-α (TNF-α) (Figure 5-6). In some mammals, especially rodents, cattle, sheep, and horses (but not in humans, pigs, goats, or rabbits), microbial PAMPs trigger macrophages to synthesize inducible nitric oxide synthase (iNOS or NOS2). This enzyme acts on L-arginine using NADPH and oxygen to produce large amounts of nitric oxide (nitrogen monoxide, NO) and citrulline (Figure 5-7). Nitric oxide alone is not highly toxic, but it can react with superoxide anion to produce potent oxidants such as peroxynitrite and nitrogen dioxide radical.
Innate Immunity
Macrophages and Recovery from Inflammation
Macrophages
Structure
Life History
Functions
Sentinel Cells
Phagocytosis
Generation of Nitric Oxide
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Innate Immunity: Macrophages and Recovery from Inflammation
Only gold members can continue reading. Log In or Register to continue