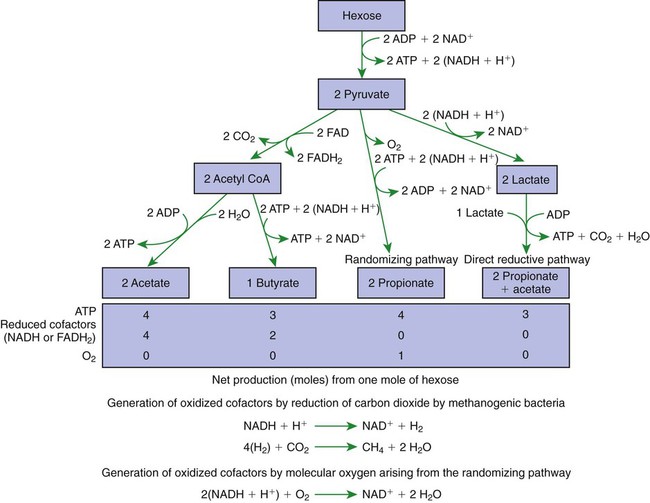
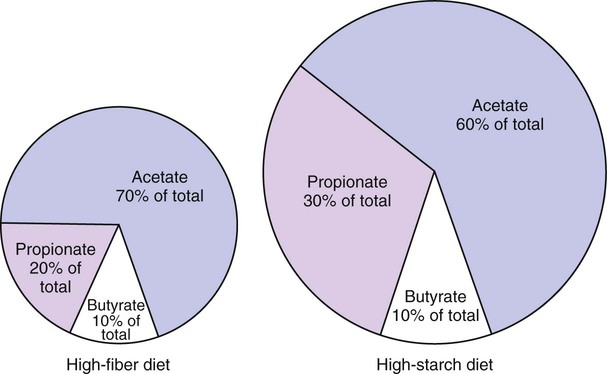
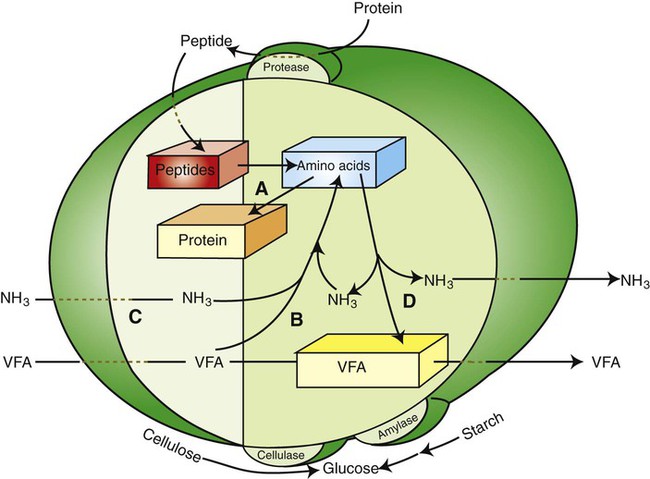
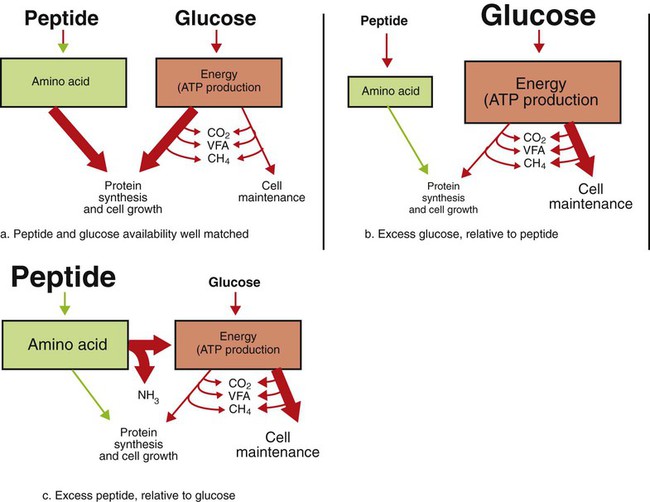
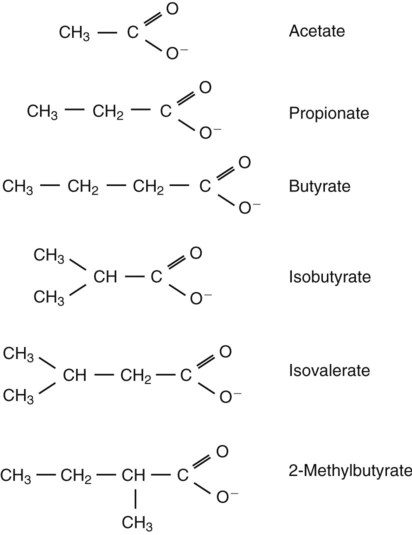
1. Fermentation is the metabolic action of bacteria. 2. The sites of fermentative digestion must be conducive to microbial growth. Microbial ecosystem of fermentative digestion 1. The microbes responsible for fermentative digestion include bacteria, fungi, and protozoa. 2. Cooperation and interplay among the many species of microbes give rise to a complex ecosystem in the forestomach and hindgut. Substrates and products of fermentative digestion 1. Plant cell walls are important substrates for fermentative digestion and significant nutrient sources for many species. 2. Nutrients other than cell walls are also subject to fermentative digestion. 3. Anaerobic conditions in the rumen result in metabolic activities leading to the production of volatile fatty acids. 4. Volatile fatty acids are important energy substrates for the host animal. 5. Fermentative digestion of protein results in the deamination of a large portion of amino acids. 6. When protein and energy availability in the forestomach are well matched, rapid microbial growth and efficient protein utilization result. 7. Microbial protein can be synthesized in the rumen from nonprotein nitrogen sources. Reticulorumen motility and maintenance of the rumen environment 1. The physiological functions of the reticulorumen maintain an environment favorable to fermentation patterns that are beneficial to the host. 2. Rumen fermentation is maintained by selectively retaining actively fermenting material while allowing unfermentable residue to pass on to the lower digestive tract. 3. Digestibility and physical characteristics of feed have important influences on both the rate of particle passage from the rumen and the rate of feed intake. 4. Rumination, or cud chewing, has an important effect on the reduction of particle size and the movement of solid material through the rumen. 5. Water moves through the rumen at a much faster rate than particulate matter. 6. Rumen dilution rate has important influences on fermentation and microbial cell yield. Control of reticulorumen motility 1. Reticulorumen motility is controlled by the central nervous system and affected by intraluminal conditions. 1. Passage of material from the reticulum to the omasum occurs during reticular contraction. Volatile fatty acid absorption 1. Volatile fatty acids, representing 60% to 80% of the energy needs of the animal, are absorbed directly from the forestomach epithelium. Rumen development and esophageal groove function 1. Significant changes in forestomach size and function occur with dietary changes in early life. 2. The esophageal groove diverts the flow of ingested milk past the forestomach and into the abomasum. Function of the equine large hindgut 1. The equine hindgut has a great capacity for fermentation. 2. The types of substrate and fermentation patterns are essentially identical for forestomach and hindgut fermentation. 3. The motility functions of the cecum and colon retain material for fermentation and separate particles by size. 4. The rate of fermentation and volatile fatty acid production in the equine colon is similar to that in the rumen. 5. Hindgut anatomy and function vary greatly among the many species of veterinary interest. The bacterial population associated with fermentative digestion is vast, with at least 28 functionally important species occurring in the rumen. Box 31-1 lists some of the major species found in the rumen and their preferred substrates. Total bacterial numbers in the forestomach or hindgut normally range from 1010 to 1011 cells per gram of ingesta. Most of these bacteria are strict anaerobes that cannot survive in the presence of oxygen, although facultative organisms are also present. In the rumen, fungi are present, and research suggests that fungi may play an important role in the digestion of plant cell walls. In fermentative digestion, pyruvate can act as an electron sink, being further reduced to provide for regeneration of NAD and the general removal of excess electrons, with an additional yield of ATP. Also, carbon dioxide can be reduced to methane, accepting electrons for the regeneration of NAD. Figure 31-1 illustrates the metabolic pathways of these reactions. These pathways lead to the major end products of the fermentative digestion of carbohydrate, the volatile fatty acids (VFAs). The primary VFAs are acetic acid, propionic acid, and butyric acid; the VFAs are often referred to as their dissociated ions: acetate, propionate, and butyrate, respectively. Other quantitatively minor but metabolically important VFAs are valeric acid, isovaleric acid, isobutyric acid, and 2-methylbutyric acid. Figure 31-2 shows the chemical structures of the VFAs. Production of propionic acid from pyruvate results in the efficient regeneration of NAD with no net production of NADH. In fact, production of available oxygen by the randomizing branch of the propionic acid pathway leads to oxidation of excess NADH originating from the acetic or butyric acid pathways (see Figure 31-1). The production of acetic acid leads to the efficient generation of ATP but, in contrast to the production of propionic acid, does not result in the regeneration of NAD from NADH. In the acetic acid pathway, excess NADH is produced. In this case, NAD is regenerated by the formation of free hydrogen, which is subsequently used to reduce carbon dioxide to methane and water (see Figure 31-1, lower portion). Thus a direct relationship exists between acetic acid production and methane production; as the amount of pyruvate entering the acetic acid pathway increases, there must be a concomitant rise in methane production. Likewise, a reciprocal relationship exists between methane production and propionic acid production; as pyruvate is diverted to propionic acid production, there is less need for methane synthesis. These relationships are shown in the stoichiometric equations of Box 31-2. These reactions do not, however, fully describe the flow of hydrogen, or reducing substances, in rumen or colonic metabolism. The chemical reactions of fermentation are extremely complex and interdependent, and NADH can donate its electrons to reactions other than those described in Box 31-2, such as the synthesis of microbial protein and the saturation of unsaturated fatty acids. The proportional rates at which acetic acid, propionic acid, and butyric acid are produced are reflected in their relative concentrations in the rumen fluid. The relative concentrations of the VFAs have important nutritional and metabolic consequences, and although seldom measured for medical purposes, VFA concentrations are frequently reported in research literature. Typically, the ruminal acetic/propionic/butyric acid concentration ratio in ruminants ranges from 70 : 20 : 10 for animals eating high-forage diets to 60 : 30 : 10 for animals eating high-grain diets. One must remember that these values represent relative proportions and not absolute amounts. The total amount of VFA produced with a high-starch diet is usually much higher than that produced with a high-fiber diet, such that total acetic acid production may be higher with a high-starch diet than with a high-fiber diet, even though the acetic acid production relative to the other VFAs may be reduced. Figure 31-3 illustrates this principle. One can appreciate the elegance and beauty of the symbiotic relationship represented by fermentative digestion by considering the metabolism of VFAs. These molecules are the end products, indeed, the waste products, of anaerobic microbial metabolism, just as carbon dioxide is the waste product of aerobic metabolism. If the VFAs were allowed to accumulate, they would suppress or alter the fermentative process by lowering the pH of the gut or forestomach. However, the host animal maintains conditions for fermentation both by buffering pH changes and by removing VFAs from the gut by absorption. The benefit derived by the host is from the chemical energy that is contained in the VFAs. These bacterial “waste products” represent spent compounds within the framework of the anaerobic fermentation system, but they still contain considerable energy that can be derived from aerobic metabolism. In ruminants and other large herbivores, the VFAs are the major energy fuels, to a large extent serving the role played by glucose in omnivorous monogastric animals. The metabolic fates of the VFAs are discussed further in Chapter 32. To this point, the discussion of fermentative digestion has centered primarily on carbohydrates, but as previously mentioned, other energy-yielding substrates are subject to microbial attack as well. Proteins are particularly vulnerable because they are composed of carbon compounds that can be further reduced to provide energy for anaerobic microbes. As proteins enter fermentative areas of the gut, they are attacked by extracellular microbial proteases. The majority of these enzymes are “trypsin-like” endopeptidases that form short-chain peptides as end products. These peptides are formed extracellularly and are absorbed into the microbial cell bodies, much as glucose is formed from carbohydrate and then absorbed. Within the microbial cells, the peptides can be used to form microbial protein or can be further degraded for the production of energy through the VFA pathways (Figure 31-4). Equation 1 always balances, but the distribution of products varies according to the relative concentrations of substrates, as illustrated in Figure 31-5. For microbial cells to be produced, both energy and nitrogen are required. Energy can come from either glucose or peptide, but nitrogen must come from peptide. When glucose and peptide availability are appropriately matched (Figure 31-5, A), energy for cellular growth comes primarily from glucose, with peptides directed toward microbial protein synthesis. Under these conditions, the products of Equation 1 favor microbial cells with little ammonia production. Glucose fermentation with accompanying VFA production must be high to meet the large energy demands necessary to support the rapid growth of the microbial mass. Ammonia production is low because most peptide nitrogen is being incorporated into microbial protein.
Digestion
The Fermentative Processes
Microbial Ecosystem of Fermentative Digestion
The Microbes Responsible for Fermentative Digestion Include Bacteria, Fungi, and Protozoa
Substrates and Products of Fermentative Digestion
Plant Cell Walls Are Important Substrates for Fermentative Digestion and Significant Nutrient Sources for Many Species
Anaerobic Conditions in the Rumen Result in Metabolic Activities Leading to the Production of Volatile Fatty Acids
Volatile Fatty Acids Are Important Energy Substrates for the Host Animal
Fermentative Digestion of Protein Results in the Deamination of a Large Portion of Amino Acids
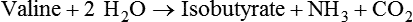
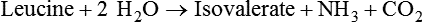
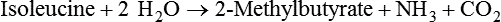
When Protein and Energy Availability in the Forestomach Are Well Matched, Rapid Microbial Growth and Efficient Protein Utilization Result
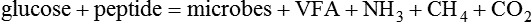 (1)
(1)
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Digestion: The Fermentative Processes
Only gold members can continue reading. Log In or Register to continue