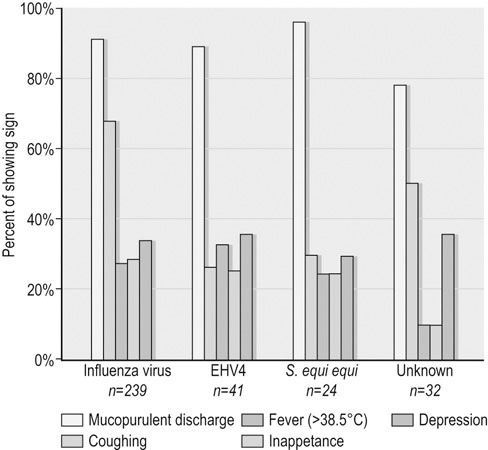
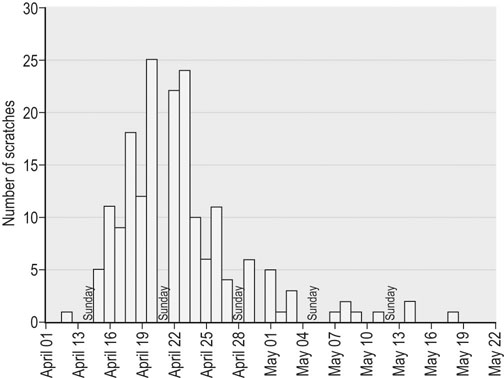
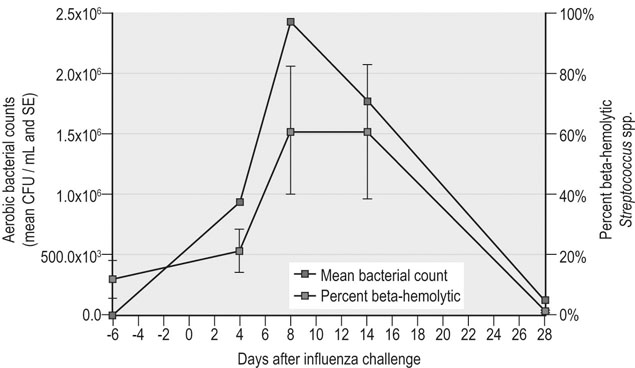
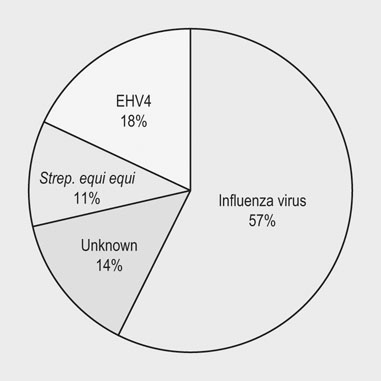
Infectious respiratory disease (IRD) is one of the most common reasons that athletic horses are removed from scheduled training and performances.1–3 Interestingly, it has been suggested that IRD is also a more frequent cause of performance disability among human athletes than all other diseases combined.4–7 Even mild respiratory disease can affect equine athletes such they are not able to attain peak performance. Most clinical occurrences of acute IRD in horses are primarily if not solely attributable to viral respiratory infections.8 While bacteria and mycoplasma can be primary pathogens, bacteria can be more important in exacerbating clinical disease after a primary viral insult. Major exceptions would be disease caused by Streptococcus equi subsp equi and Rhodoccus equi, although the latter is only a clinically important problem in foals. The importance of respiratory disease among athletic horses is generally well recognized in the equine industries. In 1997 the United States Department of Agriculture (USDA) performed a survey to identify concerns that were of highest priority to the horse industry in the United States.9 This needs assessment survey showed that problems in horses related to the respiratory system were the third most important health problem reported by all survey respondents (n = 2599), respiratory disease was the top health concern for respondents which used horses primarily for racing, and a majority of respondents considered respiratory disease agents, collectively (e.g., influenza virus, herpesvirus, etc.), to be their highest concern among infectious diseases.9 A survey of 1200 equine practitioners in the United States found that veterinarians considered viral respiratory disease second only to colic in importance among medical problems of horses.10 In addition to affecting the success of individual athletic performances, outbreaks of acute infectious respiratory disease can disrupt entire athletic competitions with important economic consequences. For example, dramatic outbreaks have been seen when novel strains of influenza virus are introduced into naïve populations (Fig. 30.1). The worldwide importance of viral IRD is further substantiated by the classification of equine influenza, equine rhinopneumonitis (herpesvirus), and equine viral arteritis as List B diseases for equids by the Office International des Epizooties (OIE) because they are considered to have important effects on international trade of animals and animal products. Firstly, equine athletes are typically young and often do not have the fully developed immunity to infectious agents that is acquired through repeated exposure to agents that commonly affect horses. Secondly, athletic horses are often congregated in large groups at training and performance facilities which increases the probability of introduction and exposure to novel contagious pathogens. Thirdly, intensive management of equine athletes at training facilities increases the probability of exposure through management practices such as the shared use of tack and other equipment, as well as through increased exposure via respiratory aerosols and contaminated surfaces. Fourthly, environmental conditions encountered by equine athletes can impair non-specific clearance mechanisms through respiratory exposure to noxious chemicals (e.g. ammonia), dust, fungi, and molds. Drying and irritation of mucous membranes caused by airflow across their surfaces have been proposed as factors affecting non-specific immunity in human athletes,11 and also might impact the health of equine athletes. In addition, racehorses are known to inhale significant quantities of small dirt particles when racing on dirt surfaces. These respiratory irritants can exacerbate non-infectious respiratory problems such as recurrent airway obstruction (heaves), which in turn are strongly believed to be a predisposing factor in viral respiratory infections in humans.12–13 This is supported by the observation that the amount of airborne particulate matter in stabling environments significantly affects airway inflammation during EHV1 infection.14,15 Infected horses stabled in barns with adequate ventilation and lower air particulate counts have less lower airway inflammation when compared with infected horses stabled in less suitable environments. The frequency of IRD among equine and human athletes can also be impacted by important effects that exercise has on immune function. Physical stresses related to athletic activity have been repeatedly shown to be associated with transient changes of in vitro and in vivo measures of systemic and local immune function. This includes changes in catecholamine and cortisol release, neuropeptide hormone release, cytokine release and activity, secretion of mucosal antibody, and the functional activity of blood and pulmonary leukocytes.16–23 These documented changes provide a basis for the ‘open window’ model proposed to describe cumulative effects of exercise on the immune system in humans.16,24,25 According to this theoretical model, moderate exercise stimulates immune function during the event and for a short time after. In contrast, heavy exertion is related to an initial stimulation followed by a longer lasting (i.e. hours) depression in cell-mediated immune function. It is proposed that during this ‘open window’ of immune depression athletes have an increased risk of becoming infected with a variety of pathogens. Since elite athletes often train intensively on a daily basis, the cumulative time that they are at increased risk of infection is considerable.16,25,26 In an effort to summarize the relationship between exercise and infectious disease, it has been proposed that the apparent relationship between intensity of exercise and its predisposing effect on the risk of upper respiratory tract infections in humans fits a J-shaped curve.16,25 This model suggests that the risk of IRD in athletes is attenuated by moderate physical activity, but is exacerbated by chronic, intensive athletic activity. While similar research in horses is not as comprehensive, the limited work that is available suggests that similar effects can be found in horses.27–37 This relationship between exercise and immune function is obviously not simple, and is likely modulated by several other factors such as likelihood of exposure to novel pathogens, lack of adequate rest, mental stress, transport, inappropriate nutrition or weight loss, and concurrent infectious or non-infectious disease. This is probably one of the reasons that studies directly evaluating the relationship between exercise and the occurrence of infectious disease in humans have met with equivocal results, even though the effects on various markers of immune function have been repeatedly and convincingly demonstrated.38 Unfortunately, almost no information is available directly examining the effects of athletic activity on the risk of IRD occurrences in horses. While IRD is very common among all horses there are few comprehensive estimates of the frequency of disease. There are two published estimates of IRD incidence in equine athletes that were obtained through prospective monitoring of racehorse populations.2,39 A study performed at a single Canadian racetrack where investigators monitored disease occurrence during two race seasons estimated that the incidence of undifferentiated IRD was about 49 IRD cases per 1000 horse-months.39 A prospective study of a cohort of Thoroughbred horses in Australia estimated the incidence of reported coughing and nasal discharge (infectious or non-infectious) to be approximately 1.8 cases per 1000 horse-months.2 It is not clear if the difference in observers explains some of the difference between these estimates (reported by research investigators versus owner reported), or if the intensive nature of horse stabling at North American racetracks compared to more extensive management of racehorses elsewhere is partially responsible for differences between these two estimates. The absence of influenza virus as a disease agent in Australia at the time of the study could have also affected these estimates. The largest population-based study estimating the frequency of undifferentiated IRD occurrence among horses was conducted by the USDA as part of the NAHMS Equine ’98 study.40 While the population for this study of owner-reported disease specifically excluded horses stabled at racetracks, it did include racehorses that were stabled at other facilities. However, it should be noted that this data includes respiratory disease occurrences from horses of all ages, including those less than one year old. An estimated 1.5 percent (SE = 0.2) of horses per quarter developed IRD during this study, and the rate of reported IRD among racehorses housed off-track was nearly three times greater than the average for all horses (4.3 percent of horses per quarter, SE = 1.9). The occurrence of IRD was reported to vary by season, and disease incidence was greatest in the spring (March through May, 2.6 percent of horses, SE = 0.6), and least common during the winter (December through February, 0.8 percent of horses, SE = 0.2). In a smaller, but similar longitudinal study, data regarding the occurrence of disease was collected from randomly selected horse operations in Michigan, and the estimated incidence of undifferentiated respiratory disease was 33 cases per 1000 horse-years.41 Horses with IRD generally develop similar clinical signs regardless of which primary or secondary agents are causing clinical disease.8 Disease is most frequently typified by the occurrence of mucopurulent nasal discharge and sometimes coughing. In a three-year study of respiratory disease at a Canadian racetrack, regardless of etiology, 80–95% of affected horses developed mucopurulent nasal discharge, while the occurrence of coughing was less common and more variable (30–75% coughed depending upon the etiology; Fig. 30.2).8 In two more recent surveillance studies enrolling randomly selected horses with acute onset of upper respiratory tract infections, fever, coughing and nasal discharge varying from serous to mucopurulent were amongst the most common reported clinical signs.42,43 Increased serous nasal discharge may be noted prior to becoming more mucoid. Paroxysmal coughing in a majority of affected animals may be suggestive of influenza virus infection, but this is not always predominant during influenza outbreaks, especially if clinical disease is mild. Fever (rectal temperature >38.5°C or 101.5°F) is less commonly noted in affected animals (10–30%).8 Abscessation and dramatic enlargement of submandibular lymph nodes can sometimes allow differentiation of animals infected with Streptococcus equi subsp. equi, but not all infected animals develop this pathognomonic sign.8,43 Many horses infected with viral respiratory agents remain asymptomatic, especially if they are older and are more immune. Others might only show vague signs such as lethargy, decreased appetite, and sub-optimal performance. Evaluation of horses with mild signs and sub-optimal performance can reveal evidence of lower respiratory tract infections (visible airway inflammation, inflammatory cytology, recovery of bacterial agents such as S. zooepidemicus subsp zooepidemicus). It is not clear if viral agents play a major role in less acute lower respiratory disease. However, seroconversion to equine herpesvirus was found to be associated with occurrences of less severe lower airway inflammatory disease, in addition to the isolation of S. zooepidemicus subsp. equi and S. pneumoniae.44 While most acute viral respiratory infections have been thought to primarily or solely affect the upper respiratory tract, the lower respiratory tract can also be affected during acute viral respiratory infections.45 Ultrasound examinations showed that horses either experimentally and naturally infected with influenza virus can develop substantial areas of pulmonary consolidation and peripheral (pleural and subpleural) irregularities.45 However, it is not clear if these lesions are principally caused by viral damage, or if this is a result of viral–bacterial co-infection. The bacterial flora of the upper respiratory tract change dramatically after viral infections, and this is principally characterized by proliferation of beta-hemolytic streptococci (Fig. 30.3; PS Morley, KW Hinchcliff, RD Slemons, DK Gross, unpublished data).46 However, it is not clear whether this change is simply a result of viral mucosal damage and solely coincidental with clinical disease, or whether this change contributes significantly to clinical disease in horses with IRD. Pneumonia is a well-recognized sequela to severe viral respiratory infections in humans, and has also been reported after introduction of novel influenza viruses to naïve equine populations.28,47–50 However, this is an infrequently recognized complication of most viral respiratory infections in horses. Interestingly, the significant pulmonary consolidation that was seen after severe experimental influenza virus infections resolved uneventfully without any treatment, even in horses that continued to exercise after infection.45 There are few published results from objective investigations of clinical parameters in horses with viral IRD. Horses infected with influenza virus have been shown to have increased resting respiratory rates and heart rates which coincided with the occurrence of fever.45 Pulmonary auscultation of horses experimentally and naturally infected with influenza virus often develop abnormal lung sounds, which was most frequently characterized as wheezing (DK Gross, PS Morley, K Hinchcliff, RD Slemons, unpublished data).45,51,52 Other systemic disease signs are sometimes reported in association with viral respiratory infections. These signs are perhaps most commonly recognized in association with equine herpesvirus type 1 (EHV1) infection; abortion and neurologic disease are well recognized in association with EHV1 infections. While essentially all EHV1 infections are thought to originate in the respiratory tract, signs of respiratory disease are not always evident before abortion or the onset of neurologic disease. While equine arteritis virus is considered an infrequent cause of respiratory disease, it has also been associated with edema of the head, legs, prepuce and abdomen, and abortion. Other systemic signs including myocarditis, rhabdomyolysis, and purpura hemorrhagica, have been reported as possible sequela of influenza virus infections. A recent study determined cardiac troponin I concentrations in ponies experimentally challenged with equine influenza virus (A/eq/Kentucky/91).53 The study demonstrated no evidence of severe myocardial necrosis secondary to equine influenza virus challenge with 108 EID50, but transient increase in cardiac troponin I suggested that mild myocardial injury occurred. This is consistent with the fact that influenza virus is strongly epitheliotropic and that viremia is not a recognized feature of influenza virus infections.50 While myocarditis has by recognized in humans in association with viral infections, this condition is more likely to be identified in association with Parvo B19, enteroviruses, adenoviruses, or cytomegalovirus, and is very rarely found in association with influenza virus infections.54 It seems likely that myocardial damage or purpura hemorrhagica found in association with IRD would result from disseminated streptococcal infection in horses rather than viral respiratory infections. Routine hematologic analysis should routinely be performed in horses with respiratory signs in order to distinguish between an infectious and a non-infectious process. It must be emphasized that the timing of blood sampling and carefully standardized laboratory techniques are essential if interpretation of leukocyte patterns, in relation to viral infections, is to be useful as a diagnostic aid. It is also necessary to be aware of the various physiological (age, breed, diurnal variation, exercise) and pathological (stress) states which can influence the total and differential leukocyte count in the horse.55 Ideally, horses with acute disease processes should be sampled during the first 24 hours of the febrile phase and because the leukocyte response is transient, sequential sampling of individual horses is necessary to detect all changes. The most consistent findings in horses with viral infectious disease of the respiratory tract are initial lymphopenia, mild normocytic, normochromic anemia and occasional mild thrombocytopenia.45,56,57 A decrease in neutrophils and monocytes also occurs, but the absolute values of both cell types remain within normal limits. The blood work rapidly normalizes within a few days and often a transient mild monocytosis and relative lymphocytosis can be observed following the acute onset. It is not uncommon for horses with viral respiratory tract infection to develop a mild increase in fibrinogen post-exposure as a reflection of inflammation. Viral infection is most often confirmed in horses using serology, which may be the most sensitive method available. However, even when isolation and epidemiologic data point to a single etiology for outbreaks of respiratory disease, serologic investigations found that only 72% of horses with disease thought to be caused by influenza virus infection seroconverted to this virus during epidemics, and only 36% of horses with disease thought to be caused by EHV4 seroconverted to herpesvirus.8,39 By far the most commonly recognized viral agents associated with respiratory disease in horses are influenza and EHV4. However, diagnostic laboratories do not routinely test for other types of viral infections. In addition, regardless of the etiology, most serological tests require evaluation of multiple blood samples; the first must be obtained soon after infection and at least two or more weeks later. It might not be logistically possible to obtain samples at these times or it might not be practically relevant or important to veterinarians or owners. Virus isolation is a very specific method for diagnosis of viral infection, but culture techniques need a week or more for completion and require specialized laboratory equipment and skills. Early identification and sampling of affected horses is critical for virus isolation as horses shed viruses for only a short period after infection. Isolation techniques have also been found to be quite insensitive as a diagnostic test during several field investigations.8,39,58 Because it can take weeks to obtain results, data from serology or virus isolation is often not very useful for enacting measures to control respiratory disease. As such, it would be useful to have simple tests available for rapid diagnoses of virus infections at the onset of epidemics so that control measures could be instituted. In recent years rapid detection assays have been developed in research laboratories for identification of several viral agents (e.g., antigen capture ELISA, PCR), but most of these tests are not widely available for use in disease prevention efforts. Rapid antigen detection kits for influenza virus are, however, commercially available throughout the world. While it would be useful to have rapid tests available for other equine respiratory pathogens, influenza virus probably causes a majority of IRD cases among athletic horse populations (where it is present in the world; Fig. 30.4), and it is one of the most contagious pathogens which increases the importance of early intervention efforts. Most of these rapid influenza detection assays are based upon using monoclonal or polyclonal antibodies to detect highly conserved viral antigens. Most of these commercial assays can be completed in about 15–30 minutes and several have been proved to be useful for detection of influenza virus in horses.59–63 Because they have been standardized and are sold in kits, they are more useful for a broad variety of diagnostic applications than are isolation techniques. While these tests are rapid and extremely useful, they have limitations. Optimal timing of sampling is important for antigen detection assays just as it is for virus isolation. Evaluation of nasal secretions collected from horses naturally infected with influenza virus suggests that only about one-third of clinically affected horses would be positive using the Directigen® assay (Becton Dickinson Microbiology Systems, Cockeysville, Maryland), which has been the most widely used assay in horses.59 Other rapid influenza tests might be more sensitive, but they might also be less specific, which can be very important when these tests are used as part of stringent disease control programs.55,56 It is important to note that more sensitive influenza detection assays have been shown to detect viral antigen in horses recently vaccinated with modified-live intranasal products, as well as from non-vaccinated horses that were in-contact with vaccinated horses.62 This can be particularly important to consider if both vaccination and rapid detection assays are being used in disease prevention efforts during outbreaks. Despite intensive investigative efforts, veterinarians frequently diagnose clinical IRD respiratory disease without identifying a primary etiologic agent. Potential pathogens were not identified from about 25% of IRD outbreaks investigated in England over a six-year period.63 Investigators in Kentucky were unable to determine the etiology of 34% (14/41) of IRD outbreaks investigated over a five-year period.64 Among 168 respiratory disease occurrences investigated during 1979, pathogens were identified for 44%.65 Investigators in Canada were not able to identify an etiology for 14% (32/277) of IRD cases identified during a two-year longitudinal study of respiratory disease among racehorses (Fig. 30.4).66 A recent voluntary surveillance study on 761 equids from the United States with clinical signs of acute onset of respiratory tract infection determined that 26.4% of index cases tested PCR positive for one or more of four selected common respiratory pathogens (equine herpesvirus-1/-4, equine influenza virus, Streptococcus equi subsp. equi).43 The highest detection rate was for EHV4, followed by equine influenza virus, Streptococcus equi subsp. equi and EHV1. The lack of etiological diagnosis for some IRD cases observed in these investigations is at least partially attributable to concentrating diagnostic efforts on identifying infection with agents that most frequently cause disease. It is likely that more comprehensive diagnostic efforts would identify agents in affected animals that tend to cause either less dramatic outbreaks or sporadic rather than epidemic disease (e.g. gammaherpesviruses or equine rhinitis virus). The most obvious change in the laboratory diagnosis of viral respiratory pathogens has been the appearance and increasing importance of nucleic acid (NA) amplification-based techniques, primarily the polymerase chain reaction (PCR), at the expense of traditional methods of clinical microbiology.67 The PCR has become an increasingly important tool in microbial diagnosis in recent years, mainly because of its rapidity, high sensitivity and high specificity. These superior characteristics have propelled the field of PCR-based molecular diagnostics into the arena of applied diagnostics for infectious agents. Because the number of published and offered PCR assays is steadily rising, there is a need for critical evaluation, comparison of performance, and eventually also standardization of methods to enable equine practitioners to select the optimal methodology. The sample of choice for the molecular detection of viruses associated with IRD is nasal secretions, which are generally collected from the nasal passages and/or the nasopharynx using rayon- or Dacron-tipped swabs. The use of viral transport medium for the transportation of nasal swabs is not needed for PCR detection, since nucleic acid-based assays do not rely on viability of the target pathogen. In order to increase the detection yield of viral respiratory pathogens, many molecular diagnostic laboratories offer panel testing for several viruses associated with infectious respiratory tract diseases. Collectively, PCR assays testing for the presence of equine influenza virus, EHV1 and EHV4 have shown increased sensitivity compared to antigen-capture ELISAs, conventional culture systems or both. Another advantage of molecular assays is their ability to detect non-viable virus, a situation which can occur when nasal or nasopharyngeal samples are frozen or not adequately stored and/or shipped to a diagnostic laboratory. Further, novel PCR platforms allow quantitation of DNA or RNA content in a given sample. This is of interest in order to assess the kinetics of viral shedding, to determine the infectious nature of a clinically or subclinically infected horse and the response to treatment. In a recent study of 369 horses that were present at two major horse shows and two major Thoroughbred, newly arrived horses were sampled to identify subclinical and clinical shedding of EIV, EHV1 and EHV4.68 Nasal swabs obtained from horses were tested using qPCR. On arrival at the four events the overall prevalence of EHV1 shedding was 3.3%, EHV4 shedding was detected in 1.1% of horses, and EIV shedding was found in 0.8%. In all instances EHV1 was detected at very low levels, and detection of EHV1 and EHV4 in nasal swab samples was not associated with clinical disease. EIV was detected only in horses sampled at a Thoroughbred sale and was associated with an outbreak of clinical respiratory disease but EHV1 and EHV4 shedding were not associated with respiratory disease. Horses under two years of age had a significantly greater risk of shedding EHV1 compared to older horses. Although rarely utilized in most horses with viral IRD, information obtained from radiography, ultrasonography, and endoscopy may be useful for evaluating the extent and nature of guttural pouch and lower respiratory tract involvement. However, there is little published literature regarding findings of these ancillary diagnostics when applied in horses infected with various viral respiratory agents. Horses have been shown to developed marked consolidation after experimental infection with influenza virus which resolved without treatment or complication.45 Similar findings have also been observed by these investigators in naturally infected horses. Endoscopic examination of these experimentally infected horses showed mild erythema and inflammation of the pharynx and airways, with increased amounts of mucoid respiratory secretions. Transtracheal wash fluid collected from horses infected with influenza virus was found to have increased cellularity and marked neutrophilia when compared with pre-infection values.45 These changes were most severe for a week after infection, but changes could be detected for much longer. Intracellular bacteria were noted in about 25% of horses for up to 14 days after infection Almost all viral respiratory infections are self-limiting and the primary therapeutic aim is to provide supportive care. Care should be taken to ensure that affected horses have access to clean water and adequate quantities of palatable feed as infected horses have been shown to lose weight and body condition.45 Providing stabling in areas with minimal dust and ammonia exposure tends to lessen airway irritation and decrease the likelihood of secondary bronchitis and pharyngitis which can limit athletic performance. It is often recommended that athletic horses with typical IRD be rested for an extended period after illness, sometimes for several weeks. This is intended to reduce the risk of serious complication such as pneumonia, pleuritis, and exercise limiting reactive airway disease. However, there are no published reports documenting the efficacy of this practice. One investigation specifically investigated whether moderate exercise exacerbated clinical disease in horses experimentally infected with influenza virus.45 This study found that all of the horses developed pneumonia after infection and that there was an exacerbated loss of body condition and mild differences in resting heart and respiratory rates among exercised horses. However, all of the horses fully recovered without treatment according to parameters investigated, regardless of whether they were rested. It should be noted, however, that this study evaluated a small number of horses and did not specifically evaluate performance parameters that might be important for equine athletes. Horses with uncomplicated viral respiratory infections will likely recover without drug therapy; use of anti-inflammatory drugs and other non-specific treatments may reduce fever and make affected animals more comfortable, but it is not clear whether they alter the course of disease. Severely affected horses might benefit from antimicrobial therapy as the bacterial flora of the respiratory tract changes dramatically during viral infections (PS Morley, KW Hinchcliff, RD Slemons, and DK Gross, unpublished data).8 Bacteria can be recovered from the lower airways of horses that exhibit signs of primary viral IRD,44,69,70 but it should be noted that there are no published studies evaluating the ability of antimicrobial (antibacterial) drugs to alter the course of IRD in horses with primary viral infections. Even horses with documented pulmonary consolidation and pneumonia secondary to experimental influenza infections were shown to recover uneventfully without the use of antimicrobial drugs.45 Most horses affected by viral IRD show marked clinical improvement within a week of the onset of disease. Ongoing respiratory disease may reflect complications related to more severe pulmonary involvement, and these horses should be evaluated using ancillary diagnostics including pulmonary ultrasound and cytology and culture of transtracheal wash fluids. Specific antiviral therapies for equine influenza virus and equine herpesvirus have been evaluated in equine and mouse models. However, none are licensed in the United States for this use in horses. In addition, considering the self-limiting nature of viral IRD, cost alone would preclude their use in most horses, regardless of safety or efficacy. In addition, use of antiviral drugs in humans is known to select for resistant strains. Amantadine and rimantadine have been used in humans infected with influenza virus, and both have demonstrated in vitro activity against equine influenza virus.51,63 Oral administration of rimantadine has been evaluated in a small number of horses and administration of 30 mg/kg, PO, q 12 h for four days was associated with amelioration of clinical signs.51
Viral respiratory disease in athletic horses
Importance of viral respiratory infections in athletic horses
How big is the problem?
Why do equine athletes have an increased risk of viral infections?
How common is infectious respiratory disease in horses?
Recognizing infectious respiratory disease
Clinical signs
Laboratory diagnosis
Hematology
Serology
Virus isolation
Rapid diagnostic tests
Ancillary diagnostic procedures
Treatment and prognosis for viral respiratory disease in horses
Rest
Antibacterial and antiviral agents
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Viral respiratory disease in athletic horses