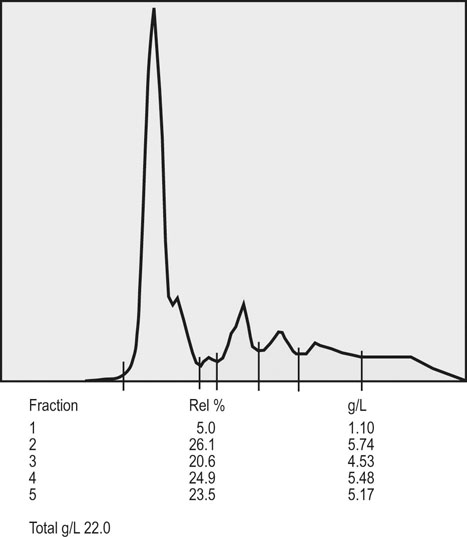
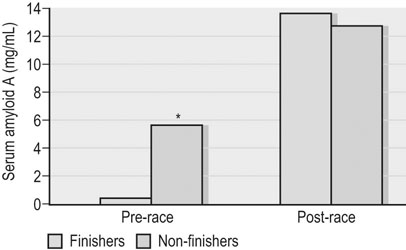
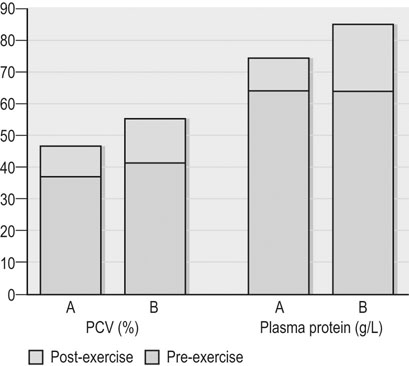
Clinical pathology, including serum or plasma biochemical analyzes, plays a pivotal role in the assessment of athletic horses with suspected poor performance or other disorders.1 Given the finely tuned nature of the performance athlete, it is not surprising that even relatively minor clinicopathologic anomalies might reflect processes capable of substantially reducing the potential for athletic success. However, since both training and exercise can also influence a variety of serum biochemistry variables in a somewhat predictable manner, the clinician must be familiar with expected biochemistry changes in the equine athlete to more accurately determine when disease might be present.1 In general, values that fall outside appropriate reference limits, or that are substantially different from previous measurements in the same individual, or which are accompanied by additional indications of disease, are likely to carry the greatest clinical significance. Obviously a wide array of disease situations can promote abnormal serum or plasma biochemistry results in horses, hence in this chapter, emphasis will be placed on serum or plasma biochemistry changes that hold particular relevance for the performance horse.2 Methods of sample collection for biochemical analyzes are described in the preceding chapter, and as previously noted, exemplary sample handling techniques are essential to avoid errors of interpretation.2 Blood samples should be centrifuged within 30–60 minutes after collection, and the separated serum or plasma ideally stored at 4°C until the time of analysis. Where in-clinic analyzes are routinely performed, the equipment should be properly maintained by frequent assessment and calibration. The staff performing the analyzes should be adequately trained in all relevant procedures, and participation in external quality control programs is necessary to ensure consistent provision of accurate results.3 Professional laboratory services provide a convenient and reliable means of biochemical analysis in non-urgent situations, provided samples are appropriately prepared, stored and transported as described in the preceding chapter. Prior communication with the receiving laboratory will often elicit valuable information regarding sample collection and submission, further reducing the potential for preparation or handling errors to affect the quality of the obtained results.2,3 Currently over 1000 different plasma proteins have been characterized; however, only a minute fraction are individually measured on a routine basis in equine plasma.3 Specific proteins that can be useful indicators of systemic inflammation or disease are broadly categorized into three groups; positive acute phase proteins, negative acute phase proteins, and delayed response proteins.4 Positive acute phase proteins are those which increase in concentration in response to an inflammatory process, and include fibrinogen, serum amyloid A (SAA), ferritin, and haptoglobin. Negative acute phase proteins, such as albumin and transferrin, decrease in concentration when inflammation is present, usually due to loss from the body or vascular space, or reduced production, respectively. Delayed response proteins are those which increase in concentration one to three weeks after the onset of inflammation, and include the various immunoglobulin fractions (immunoglobulins A, G, M, and E) and the complement proteins.3,4 Dysproteinemia is characterized by abnormal concentrations of total protein, albumin or globulin in the serum or plasma. It should be recognized that plasma total protein and serum total protein differ quantitatively due to the absence of fibrinogen in the latter, and that ‘globulin’ represents the cumulative total of all plasma proteins excluding albumin.3 Serum protein electrophoresis (SPE) can be used to more accurately qualify dysproteinemia situations by separating out protein fractions based on their migration characteristics through a medium to which an electrical field is applied. Typically, equine total serum protein will separate into a large albumin fraction, representing approximately 50% of the serum protein content, with the remaining fractions comprising the alpha 1-globulins, alpha 2-globulins, beta 1-globulins, beta 2-globulins and gamma-globulins (Fig. 43.1).3–5 Alterations in SPE morphology associated with a variety of pathologic conditions have been described in horses.4,5 In general, however, this procedure has limited diagnostic value and should be utilized in conjunction with additional diagnostics appropriately tailored to the clinical signs or disease process of concern.3 Simple quantitative measurement of total protein and albumin concentrations in performance horses can provide useful information regarding infectious or parasitic disease. Hypoproteinemia resulting from low blood albumin concentrations most frequently reflects some form of gastrointestinal disease in horses. Given that many performance horse populations are composed of young, potentially immunosuppressed horses exposed to variable extremes of management and crowding, gastrointestinal parasitism should be considered among the likely possible causes of hypoalbuminemia in young horses, particularly if history or inspection suggests they are dwelling in suboptimal management situations.6 In addition, protein losing enteropathy associated with Lawsonia intracellularis infection is most prevalent in weanling Thoroughbred and Standardbred horses destined for performance careers, potentially also reflecting the intensive management of some performance horse operations.7,8 Lawsonia infection in horses is characterized by moderate to profound hypoproteinemia which is variably associated with additional anomalies including ventral edema, diarrhea, fever, neutrophilia, hyperfibrinogenemia and serum electrolyte disturbances.7,8 Other potential causes of hypoproteinemia that are possibly relevant to the performance horse include administration of non-steroidal anti-inflammatory drugs, sand ingestion, severe gastric ulceration, and infectious disease situations including pleuropneumonia, which occurs with a greater prevalence in racing Thoroughbred horses compared to other equine populations.9–11 Given the nature of the diseases that can cause protein loss in horses, evaluation of the hypoproteinemic performance horse should likely include thorough physical examination, fecal analysis for the presence of parasite ova and complete blood count analysis to identify any additional indicators of inflammation or infection including hyperfibrinogenemia, neutrophilia, and anemia. Additional diagnostic procedures should then be selected based on detailed anamnesis and the results of the physical examination and initial diagnostic tests, and may include thoracic and abdominal ultrasonography, tracheal wash procedures, gastric endoscopy, and abdominocentesis.12 If Lawsonia intracellularis infection is suspected, blood and fecal samples should be submitted for serologic assays (immunofluorescence or immunoperoxidase monolayer assay) and polymerase chain reaction analysis, respectively.7 Plasma fibrinogen represents a very commonly measured acute phase protein (APP) in equine populations.4 Fibrinogen can be accurately measured from fresh whole blood samples collected into EDTA or sodium citrate anticoagulants, but should not be measured in heparinized blood; hence, care should be taken when collecting samples for fibrinogen analysis via indwelling venous catheters to avoid heparin contamination.3 Fibrinogen is considered a positive moderate APP partly because of its slow response dynamics, taking days to weeks to peak and return to normal plasma concentrations.4 The combination of this behavior with a fairly wide reference interval in healthy horses makes fibrinogen a fairly insensitive indicator of inflammation in the horse.4 Serum amyloid A (SAA) currently represents the most sensitive and only major positive APP analyzed with frequency in horses.4 This protein is normally present in the serum of healthy horses at undetectable to low concentrations, and production of SAA by the hepatocytes increases rapidly in response to a variety of inflammatory and disease situations. Extra-hepatic isoforms of SAA also exist and can be measured locally at their sites of production (for example, measurement of SAA3 can be performed in joint fluid).4 Several methods of assay for SAA exist, including ELISA, latex agglutination and immunoturbidimetric techniques. Reference intervals also vary with the most broad reference limits currently in use suggesting values of between 0 to 20 mg/mL to be appropriate in healthy horses.4 Concentrations of SAA increase rapidly and substantially in 24 hours in response to inflammation or infectious conditions, including viral and bacterial respiratory infections, colic, surgery and experimentally induced septic arthritis.4,13 Rapid methods of quantifying equine SAA have been reported from Europe where measurement of SAA as a screening assessment in poor-performing horses or for determining fitness to return to training after an illness is anecdotally reported. However, further research to conclusively define the utility of this assay for such purposes is required.4,13,14 Nonetheless, the application of SAA measurement in athletic horse populations appears to be currently expanding. In a study of 20 endurance horses participating in 120 or 160 kilometer distance endurance races, all horses with a pre-competition SAA concentration exceeding 1000 ng/mL (1.0 mg/L) failed to complete their event, with 9 of these 12 horses subsequently removed from competition due to the occurrence of lameness conditions.15 However, plasma SAA concentrations after competition were found to be equally elevated in successful and eliminated horses, and remained below 20 mg/mL in all but one horse (Fig. 43.2).15 Similar elevations in SAA were reported in another study of endurance horses competing in races of 120 or 160 km; however, serum haptoglobin and C-reactive protein concentrations in the same horses were unchanged.16 Additionally, serum concentrations of SAA, haptoglobin, and C-reactive protein were not observed to increase significantly in endurance horses completing limited distance events (34 and 60 km).16 Collectively, these findings indicate that long-distance endurance exercise increases SAA concentrations in horses, though values usually remain within current broad reference limits. Further investigation is therefore indicated to define appropriate reference limits for SAA concentrations in healthy athletic horses, and to elucidate the discriminatory power of SAA measurement in various equine performance activities. At the onset of exercise, inter-compartmental fluid shifts promote rapid and transient increases in plasma protein and albumin concentrations in healthy horses.17 More pronounced and persistent changes are noted in horses undertaking endurance exercise, in which fluid shifts are compounded by progressive and substantial fluid losses over time, related to sweat losses which can exceed 10 L/h in a warm environment.18 Metabolic derangement associated with perturbations of fluid and electrolyte balance is one of the most common causes of elimination of horses from endurance competition.19–21 Increases in plasma protein concentration and hematocrit commonly occur in endurance horses, and to a large degree will reflect the immensity of body water losses.22–24 Therefore monitoring changes in hematocrit and plasma protein, in addition to other clinical and laboratory variables, constitutes one valuable method of potentially identifying horses at risk of metabolic derangement during or after endurance competitions. Previous reports have identified higher plasma protein, hematocrit or both in horses that are eliminated from endurance racing or which require treatment for metabolic derangements.23–25 Supporting this finding, a large-scale study of endurance horses competing in races of 72 to 169 km in length found both PCV and plasma protein concentration to provide good prediction ability for metabolic elimination.21 Plasma protein concentrations had greater sensitivity and specificity for this purpose than PCV, perhaps reflecting the confounding impact of splenic contraction on PCV. Ultimately the authors concluded that PCV values exceeding 52% and plasma protein concentrations exceeding 82 g/L were associated with the development of metabolic alterations in competing horses.21 Similar values have been reported in other studies of endurance horses competing in similar distance races, with higher PCV and plasma protein values reported in horses developing metabolic derangements during or after racing (Fig. 43.3).25,26 Hence, where possible, assessment of PCV and plasma protein concentrations should be incorporated into the assessment of individual endurance horses considered at risk of metabolic derangement during competition. Due to the relatively high prevalence of exertional rhabdomyolysis disorders in various equine athletic performance groups, including Thoroughbreds, Warmbloods, Quarter Horses and related breeds, measurement of muscle enzyme activity should be routinely included in the assessment of poor-performing horses.27,28 Muscle damage in the horse is currently most accurately reflected by an increase in serum or plasma creatine kinase (CK) activity, which peaks within 4 to 6 hours after muscle injury has occurred. However, due to its brief half-life (approximately 2 hours), serum CK activity declines rapidly if the injury is transient in nature.3 Aspartate transaminase (AST) activity peaks approximately 12–24 hours after muscle or liver injury in the horse but has a longer half-life of approximately seven to eight days.3 Therefore, interpretation of serum CK activity, combined with assessment of AST and additional liver enzyme activities (sorbitol dehydrogenase, gamma-glutamyl transferase) can accurately distinguish the source of enzyme derangements and can be a valuable means of delineating acute and chronic muscle disease in performance horses (Fig. 43.4).5
Biochemical abnormalities of athletic horses
Introduction
Clinical chemistry as an indicator of disease
Plasma proteins
Muscle enzyme activities
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Biochemical abnormalities of athletic horses