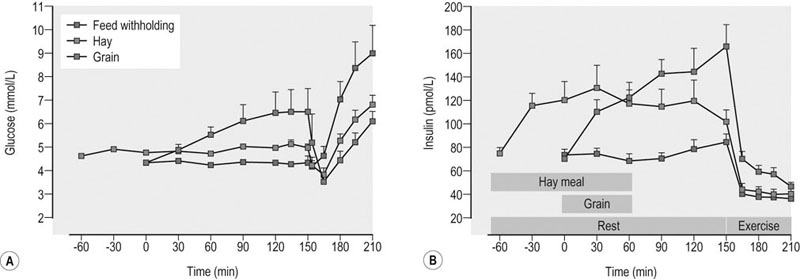
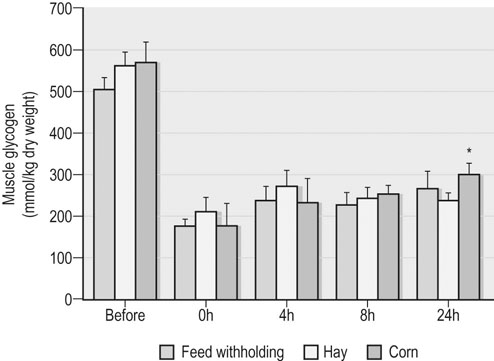
As described in Chapter 36, the main considerations in ration formulation are: 1. provision of adequate fiber (roughage) to maintain normal gut and digestive function (and to support the horse’s natural chewing behavior and thereby reduce the risk of certain behavioral disturbances) 2. targeting of an overall energy density that will allow energy requirements to be met at typical fed intakes 3. provision of the optimal amounts and balance of other essential nutrients (i.e., protein/amino acids, minerals, trace elements and vitamins) 4. use of only the highest hygienic quality feedstuffs that have been stored appropriately. In addition to these basic principles, there are a number of other topics related to the nutritional management of the athletic horse during training and competition that warrant discussion, including: • the timing of feeding before exercise, i.e. when and what should be fed before bouts of heavy exercise • electrolyte supplementation, i.e. what quantity and type of electrolytes should be given, and when • the effect of diet and post-exercise feeding strategy on muscle glycogen content • what nutritional adjustments are indicated for the management of specific problems that commonly occur in athletic horses, e.g. gastric ulcer syndrome, equine rhabdomyolysis syndrome • the potential benefits of oil (fat)-supplemented rations • whether there is any rationale for the addition of ‘specialized supplements’ to the ration of an athletic horse. The timing and composition of meals consumed before exercise may impact health and performance via effects on fluid balance, blood flow distribution, metabolism and perhaps also mechanical discomfort associated with gut fill. A full stomach may restrict the space available for lung expansion during exercise although there are no published data to support or refute this contention. Pre-exercise feeding also might alter the distribution of blood flow, with potentially adverse effects on blood flow to the respiratory and locomotor muscles. However, one study in horses during moderate exercise demonstrated no adverse effect of a pre-exercise meal on blood flow distribution when compared to the control treatment.1 Presumably the exercise-associated increases in heart rate and cardiac output were sufficient to meet the competing demands for blood flow to the splanchnic bed as well as the respiratory and locomotor muscles. Whether the same is true in regards to feeding before high-intensity exercise in horses is not known. Several studies have examined the effects of the timing and composition of a meal consumed before exercise on metabolic responses in horses.2–5 Most notably, the hyperglycemia and insulinemia associated with the digestion and absorption of starch-rich (grain) meals affects the mix of substrates utilized during a bout of exercise. Insulin is a potent inhibitor of lipolysis and fatty acid oxidation in skeletal muscle, and also promotes glucose uptake into muscle via recruitment of the transporter protein GLUT4 to the sarcolemma. Thus, hyperinsulinemia at exercise onset will suppress FFA availability and lipid oxidation and increase reliance on carbohydrate stores (including plasma glucose) for energy transduction. Accordingly, several equine studies have demonstrated that a grain meal (1–3 kg of oats, corn or a mixture of the two) consumed 3 hours or less before exercise results in hyperglycemia, hyperinsulinemia (Fig. 37.1) and decreased plasma FFA concentration at the start of exercise, and a subsequent marked decrease in plasma glucose concentration during the initial period of exercise.2–5 Jose-Cunilleras and colleagues6 utilized isotopic tracer methods and indirect calorimetry to determine the effects of hay (alfalfa cubes; ~3 kg) and starch (cracked corn; 1.7 kg) feeding on glucose flux and substrate oxidation during exercise. The alfalfa cubes were consumed between 2 and 3 hours before exercise, while the cracked corn was ingested 90 min pre-exercise. Feeding corn before exercise resulted in increased utilization of blood-borne glucose and whole-body carbohydrate oxidation when compared to a meal of alfalfa or not feeding (Fig. 37.2). In another study in which smaller amounts of forage and cereals were fed (~1 kg each) 1.5–2 h before a simulated trotting race (2600 m on a 2.5% incline at around 90% of VO2max), no differences in blood glucose and insulin values were found between the pre-exercise samples and resting pre-prandial samples.7 The effects of pre-exercise grain feeding on endurance exercise performance in horses per se have not been reported. In humans, carbohydrate ingestion during exercise unequivocally improves performance during prolonged (more than 2 hours) moderate-intensity (>50–60% of VO2max) exercise, presumably by maintaining glucose supply in skeletal muscle at a time when glycogen stores are depleted. On the other hand, the performance effects of pre-exercise glucose feedings in human athletes are more equivocal. For horses performing prolonged (endurance) exercise, the acceleration in carbohydrate oxidation (and suppression of fat oxidation) associated with pre-exercise grain feeding might impair performance as a result of carbohydrate depletion. The impact of feeding during endurance rides may be more complex as in these circumstances exercise-associated alterations in hormones (e.g., increased catecholamines, decreased insulin) may counterbalance the effect of any hormonal changes induced by feeding. One study in horses looked at the effect of feeding a grass meal based pellet with or without either 50% glucose or fructose before and during a simulated 30 km endurance ride.8 Plasma glucose concentrations were elevated during the simulated endurance exercise after glucose feeding, but no counter-regulation by insulin occurred. Plasma insulin, FFA and lactate levels showed exercise-related changes but dietary treatment did not affect these results. As demonstrated in the study by Jose-Cunilleras et al.,6 together with the results of earlier studies by Pagan and Harris,5 forage meals (<2 kg) consumed 2–3 hours before exercise have minimal effect on substrate availability and oxidation during sustained exertion. However, free choice consumption of hay in the 12–24 hour period before exercise may adversely affect performance because of an increase in body weight (gut fill), especially in horses not adapted to such a ration or when hays with a reduced digestibility are fed. Large meals (hay or grain or a combination) consumed near the start of exercise will also result in a decrease in plasma volume as a result of fluid shifts into the gastrointestinal tract.5 Such reductions in plasma volume could compromise cardiovascular function during exercise. There is some evidence that a short-term reduction in forage intake may be beneficial in horses undertaking high-intensity exercise – although this may depend on the pre-reduction intake; e.g. when compared to ad libitum hay consumption, restricting hay intake to ~1% of bodyweight for a three-day period before a treadmill exercise test (2 min at 115% VO2max) resulted in a 2% decrease in body weight and a reduction in anaerobic energy expenditure during exercise, as evidenced by reduced oxygen deficit and plasma lactate concentrations. The reduction in body weight was attributed to a reduction in gut fill.9 • Limit the size of pre-exercise grain or complementary feed meals (<1 kg/500-kg horse) and ideally do not feed this type of feed within 2–3 hours of the event. Feed <1 g/kg BW of starch and sugar in the pre-exercise meal • Provide 1–2 kg forage (500-kg horse) (e.g. in a double hay net/haylage net) 2–3 hours pre-exercise to promote slow chewing and salivation without excessive gut fill • Do not bed on straw or wood shavings during the period of feed withholding before exercise. The consumption of bedding materials (e.g. straw, wood shavings) may increase under conditions of forage restriction.10 This intake of bedding material might increase the risk of colic in some horses. There also is a potential increased risk of colic when horses are provided access to a new bedding source at a competition • The inclusion of alfalfa in the pre-exercise forage meal should be considered as some studies have suggested that alfalfa feeding may be beneficial with respect to prevention of exercise-associated gastric ulceration.11 • Avoid feed changes. A recent change in hay or forage feeding (as well as grain or concentrate feeding) is associated with increased risk for an episode of colic (the period of highest risk is 2 weeks post change).12,13 Clinical experience suggests that changing from one batch to another of the same forage type carries less risk of colic when compared to a change from preserved to fresh forage or from a forage-only diet to a mixed forage-concentrate diet. Ideally, any changes in the diet should be done gradually. • A slight reduction in training effort linked with maintenance of an appropriate diet should help to ensure that muscle and liver glycogen stores are as close to optimal as possible prior to the undertaking of competition exercise (see below). It has been suggested that the feeding of a high glycemic meal the night before competition may facilitate the ‘topping-up’ of liver glycogen stores; this strategy should only be applied in horses adapted to grain. Whether or not electrolyte supplementation has a beneficial effect on health and performance, especially during prolonged exercise, is a controversial topic in equine nutrition (see Harris and Schott14 and also Chapter 40 and Chapter 52). Electrolyte supplementation of athletic horses is a common practice and it is generally held that at least partial replacement of sweat ion losses is needed for avoidance of fluid, electrolyte and acid–base disturbances that may adversely affect health and performance. No adverse effects were detected in eventing horses that received no or ‘inadequate’ amounts of additional sodium chloride supplementation.15 As well, some elite endurance horses are apparently not provided electrolyte supplementation during rides up to 120 km in distance. Nonetheless, a study of French endurance horses suggested that in such circumstances renal mechanisms were not able to compensate for electrolyte losses during the ride.16 The authors of this study concluded that their findings supported ‘the benefit of electrolyte supply during rides’.16 Furthermore, when horses were exercised at 30% VO2max 1 hour after the administration of 3 L of an electrolyte solution, the radiolabeled sodium in the solution appeared in the sweat by 10 min of exercise and the duration of exercise to voluntary fatigue was increased by 33 ± 10%.17 These findings provide a rationale for electrolyte supplementation, particularly during long endurance rides, although questions regarding the timing, amount and composition of supplementation require additional research. The following comments are particularly relevant to electrolyte supplementation of endurance horses but the principles also can be applied to horses used in other activities that elicit substantial sweat fluid loss: • The amount of sweat produced depends on environmental conditions, nature of the work performed (which also depends on the rider’s ability and terrain) and the animal’s fitness.18 • Under mild ambient conditions, horses (~500 kg BW) may lose 5–7 liters of sweat per hour of steady trotting and cantering.19,20 However, under conditions of high heat and humidity, sweat rates may approach 10–12 liters per hour.19,21 • In addition, to the large amounts of water that can be lost in sweat by horses performing several hours of endurance exercise, equine sweat contains 110–130 mmol Na+ (2.5–3.0 g/L), 120–140 mmol Cl− (4.3–5.0 g/L), and 30–40 mmol K+ (1.2–1.6 g/L) per liter.19,20 • In theory, intake of water and electrolytes via (1) the oral administration of hypertonic electrolyte slurries followed by voluntary drinking or (2) by initially offering salt water followed by plain water, should limit dehydration and enhance recovery, but supporting data are lacking. Similarly, the effects of electrolyte supplementation on performance are not well established. There are potential risks associated with repeated electrolyte administration, especially by hypertonic pastes (e.g. increased risk of gastric ulceration) and if supplied to dehydrated animals (potential for further dehydration due to initial influx of fluid into the gastrointestinal tract). • Using a typically applied factorial approach to electrolyte replenishment that is based on body weight loss and estimated sweat electrolyte content appears to overestimate the daily requirements of horses, especially those that lose large quantities of sweat fluid. Basing salt requirements according to energy expenditure also overestimates sodium requirements and depending on the core diet may influence acid–base status.22 • A forage-based diet should provide adequate potassium, and for most animals also adequate calcium and magnesium; therefore the main concern should be for salt replenishment during training (note that low-forage, high-cereal rations may not provide sufficient calcium or potassium). • Salt should be introduced or removed from a feed gradually. • For many horses in light work, access to a salt block or free salt is likely to be sufficient. When a commercial feed or a vitamin-mineral supplement is being fed, the block should be pure salt rather than a salt-mineral type (blocks formulated for other species should not be used). However, some horses may not consume adequate salt when a salt block is the sole source. Accordingly, loose salt should be provided to horses in moderate/hard work (either added to feed or provided in a separate vessel). • During competitions, a conservative starting point for supplementation is to add 1.2 g Na per kg of sweat loss to the ration, i.e. over and above maintenance requirements. This supplemented amount represents ~30–40% of estimated losses. • The optimal level of salt supplementation likely varies between horses, and will depend on many factors during a competition including environmental conditions and the fitness of the horse. • During endurance competitions, additional potassium chloride supplementation is often provided (in a 3 : 1 ratio of NaCl : KCl) although there is some dispute whether such potassium provision should occur during loops undertaken at high intensities. • Provide supplementary electrolytes during the 24 h post-race period – the inclusion of potassium in this supplement is recommended. The timing of electrolyte supplementation pre-exercise is also controversial. The thirst response peaks around 2–3 hours after the administration of hypertonic electrolyte slurries, with urinary excretion continuing for up to 6 hours post-administration. The aforementioned study in which radiolabeled electrolytes were administered orally demonstrated the appearance of Na and K in the plasma within 10 min of administration, with peak values at 20–40 min and a more rapid clearance of K than Na from plasma.17 The authors recommend that any electrolytes should be administered at around 2 hours pre-exercise but only in animals that are fully hydrated. Fresh, clean drinking water must be available to the horse when electrolyte pastes/slurries are administered. The increased demand for energy (adenosine triphosphate, ATP) to sustain muscle contraction during exercise is matched by the increased utilization of energy substrates. The primary fuel sources within muscle are ATP, phosphocreatine (PCr), glycogen and triglycerides (TG), while the primary extramuscular fuel sources are blood-borne glucose (from hepatic glycogen and gluconeogenesis) and NEFA (from adipose TG stores). There are three immediately available energy sources in skeletal muscle: ATP, PCr and adenylate kinase (myokinase). These phosphagens have very low capacity with respect to providing fuel for contraction, and energy from glycolytic and oxidative metabolism is needed to sustain all but very brief (<20–30 s) periods of exercise.23 Muscle glycogen is the primary energy source for anaerobic glycolysis and oxidative phosphorylation during intense exercise in horses, and also is an important fuel during prolonged, lower-intensity exercise. Indeed, experimental studies have suggested that most of the energy for exercise at intensities above 50% VO2max is derived from carbohydrates (muscle glycogen, blood glucose, and lactate).24 The chemical energy required for muscle contraction is ultimately provided in the diet. Therefore, inasmuch as diet can influence the storage of glycogen and TG in muscle, there is an obvious relationship between dietary intake and skeletal muscle exercise metabolism. Studies in human athletes have unequivocally demonstrated that a high carbohydrate (CHO) and low fat diet will result in higher muscle glycogen concentration when compared to a more conventional, lower CHO diet.25 Furthermore, there is a direct relationship between initial muscle glycogen stores and performance during moderate-intensity (50–80% of VO2max) endurance exercise in humans.26 Thus, for human athletes, dietary strategies that boost muscle glycogen stores enhance exercise performance. Conversely, a low CHO and high fat diet impairs endurance exercise performance, in part due to lower initial muscle glycogen concentrations. The depletion of muscle glycogen reserves is also likely to contribute to fatigue and poor performance in high-intensity as well as endurance exercise in horses. Depletion of muscle glycogen stores has been associated with fatigue in horses during endurance racing,27 and the time to exhaustion in horses running at 6–7 mph was decreased by 35% when pre-exercise muscle glycogen content was 70% lower than in control horses.28 Anaerobic work capacity in horses during a sprint treadmill exercise test was decreased by approximately 28% when muscle glycogen content was 60–70% lower relative to a control treatment.29 Taken together, there is a rationale for feeding and training strategies that optimizes muscle glycogen content in preparation for competition exercise. Post-exercise replenishment of muscle glycogen is two- to threefold fold slower in horses than in man and other mammals.30 During the first 24 hours post-exercise, very little repletion occurs under conventional feeding conditions and full recovery after exhausting exercise may take up to 3 days. As a result, hoses that are exercised frequently (on a single day or on consecutive days) may have low muscle glycogen stores at the start of subsequent exercise bouts, which may impair physical performance. In humans, muscle glycogen synthesis following glycogen-depleting exercise follows two phases.31 Initially, there is a period of rapid synthesis that functions independent of external factors such as insulin and last about 30–60 minutes. Exercise-induced translocation of glucose transport protein-4 (GLUT4) to the sarcolemmal membrane, with increased permeability of the muscle membrane to glucose, is a key determinant of this rapid phase of glycogen resynthesis. The second phase of resynthesis is slower and lasts several hours. Systemic glucose availability, insulin stimulation of glucose uptake into muscle, and the activity of glycogen synthase (GS; the ‘rate-limiting’ enzyme in glycogen synthesis) interact to control the rate of glycogen synthesis during this phase (Fig. 37.3).31 Another important component of this slower phase is a post-exercise enhancement in skeletal muscle insulin sensitivity to glucose transport, an effect that persists for as long as glycogen stores remain below resting concentrations.32 Several factors may account for the comparatively slow rate of muscle glycogen resynthesis in horses.30 In humans, an up to six-fold increase in muscle GS activity has been observed after glycogen-depleting exercise and GS activity is further increased in response to carbohydrate feedings or IV insulin administration.33 Skeletal muscle GS activity in horses is much lower when compared to humans,34,35 only increases to a modest extent following exercise that depletes glycogen by 40–50%,34 and no further increase in post-exercise GS activity is observed in response to hyperinsulinemia35 or following starch-rich meals.36 Additionally, a post-exercise enhancement in insulin-stimulated glucose disposal (insulin sensitivity) does not occur in horses.35 Taken together, these observations suggest that glucose transport into muscle and the activity of GS restrain glycogen resynthesis in horse skeletal muscle and, at least in part, may explain why nutritional strategies that emphasize hyperinsulinemia and increased glucose availability provide only a modest enhancement in the rate of muscle glycogen resynthesis. The relatively low (with respect to horses) glycogen content of human muscle (~300 mmol/kg dry muscle [dm]) can be doubled by a combination of exercise and diet. Originally this ‘glycogen loading’ involved a 2–3 day depletion phase of hard training on a low carbohydrate diet followed by a 2–3 day loading phase of high carbohydrate intake with a tapering of exercise. It now is evident that the combination of exercise taper and high daily CHO intake (~7–10g/kg BW/day) will increase muscle glycogen storage.37 This level of starch and sugar feeding is difficult to achieve in horses and not recommended given the recognized associations between high dietary starch and risk of gastric ulcer syndrome, intestinal disturbances, etc. Several different feeding strategies for enhancement of the post-exercise rate of muscle glycogen synthesis have been evaluated in horses, with a primary focus on carbohydrate feedings (glucose, glucose polymers or starch-rich meals) largely because these approaches have proven successful in human athletes.30 The primary conclusion from this work is that feeding (or administering) glucose or starch in amounts comparable to that used with success in humans does not substantially (if at all) alter muscle glycogen recovery in horses. For example, oral administration of a glucose polymer (3 g/kg BW) within 60 min of the completion of glycogen-depleting exercise38 or 1 g glucose/kg BW given at 0, 2 and 4 h post-exercise34 did not enhance or alter the rate of muscle glycogen resynthesis in Standardbred horses (Fig. 37.4). Provision of a 80% grain (corn, oats and barley mix):20% hay diet modestly increased glycogen recovery over a 72-h period when compared to hay only or 50 : 50 grain : hay diets.36 Feeding two meals of cracked corn at 0 and 4 h after glycogen-depleting exercise (2.2 kg corn/meal, total digestible energy intake of ~15 Mcal) enhanced systemic glucose supply (threefold increase in whole body glucose kinetics) but minimally enhanced muscle glycogen replenishment at 24 h post-exercise when compared to feed withholding or isocaloric feedings of hay (grass and alfalfa hay mix) (Fig. 37.5).39 In contrast, intravenous administration of glucose (6 g/kg BW over 12 hours,40,41 or about 0.5 g/kg BW/h) was demonstrated to increase muscle glycogen recovery by 24 h post-exercise. Alternative strategies for the enhancement of muscle glycogen recovery in horses have been examined, including the addition of amino acids to glucose feedings, short-chain (volatile) fatty acid supplementation, and provision of a hypotonic electrolyte solution. Studies in humans have shown that the addition of protein hydrolysates or certain amino acids to carbohydrate/glucose feedings can enhance insulinemic response and glycogen replenishment when compared to carbohydrate alone.42 Leucine is an insulin secretatogue and, in rodent studies, has been demonstrated to increase muscle glucose uptake; both mechanisms may promote muscle glycogenesis when leucine is fed along with glucose. It should be noted that protein or amino acid supplementation does not augment muscle glycogenesis in humans when larger amounts of carbohydrate are consumed during recovery (~1.2 g CHO/kg BW/h) which is considered the optimal dosage for enhancement of glycogen resynthesis.43 In horses, the addition of 0.1 g/kg BW44 or 0.3 g/kg BW45 leucine to a glucose dose was shown to substantially increase post-exercise plasma insulin responses when compared to glucose alone. However, the administration of leucine (0.1 g/kg BW at 0 and 4 h post-exercise) in concert with glucose (1.0 g/kg BW at 0, and 4 h post-exercise) did not alter post-exercise glycogen replenishment in Standardbred horses.46 Whereas amino acid supplementation may not enhance early post-exercise glycogen recovery, recent work has suggested that protein nutrition can affect muscle glycogen content. Specifically, the muscle glycogen concentration of Standardbred trotters fed a forage-only diet with 16.6% crude protein (CP) was higher (~630 mmol/kg DM) when compared to a forage diet providing 12.5% CP (~550 mmol/kg DM), and there also was a trend for faster post-exercise glycogen recovery in horses fed the higher protein diet.47 However, the 16.6% CP hay also had greater NSC content and this difference may have contributed to the higher muscle glycogen concentration in this treatment. Low availability of lipid metabolites during the post-exercise recovery period may limit muscle glycogenesis by redirecting glucose away from glycogen synthesis to support immediate energy needs through the tricarboxylic acid (TCA) cycle,48 i.e. available glucose is partitioned to oxidation rather than storage. Therefore, post-exercise feeding strategies that directly or indirectly (e.g. via production of volatile fatty acids by microbial fermentation) increase the availability of alternative substrates for the TCA cycle (such as acetate) could support glycogen synthesis. The ingestion of a glucose/acetate solution increased glycogen replenishment and GS activity in rats after exercise when compared to glucose alone.49
Nutrition for the equine athlete
Above and beyond nutrients alone
Introduction
Time of feeding before exercise
Electrolyte supplementation
Dietary manipulation of muscle glycogen content
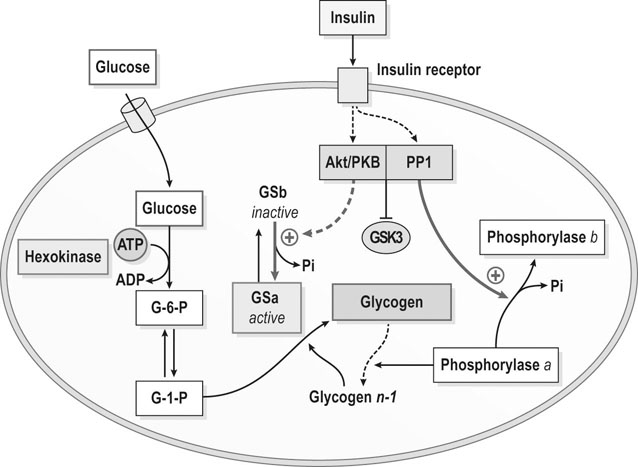
Muscle glycogen and exercise performance
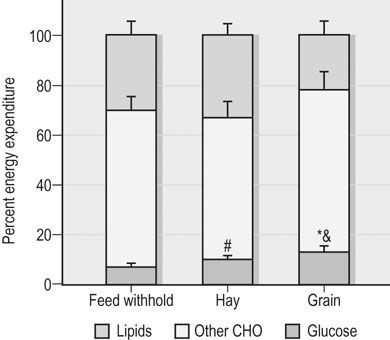
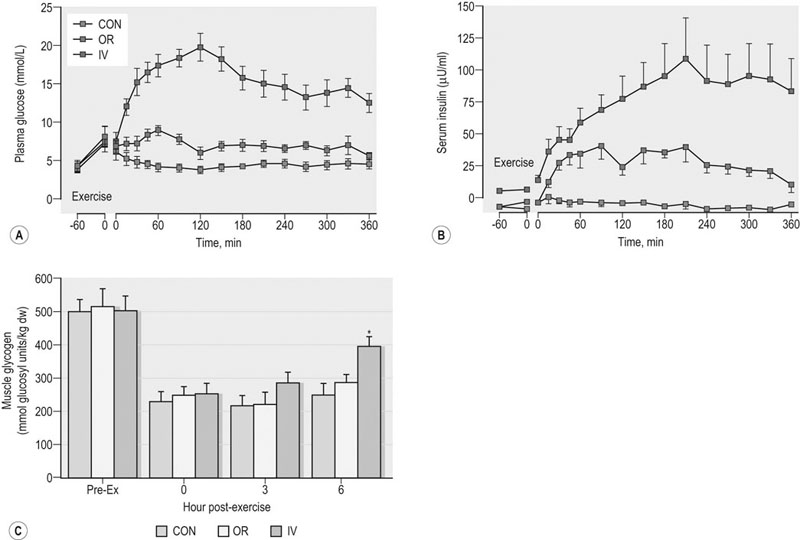
Post-exercise muscle glycogen synthesis
Diet and muscle glycogen storage
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nutrition for the equine athlete: Above and beyond nutrients alone
