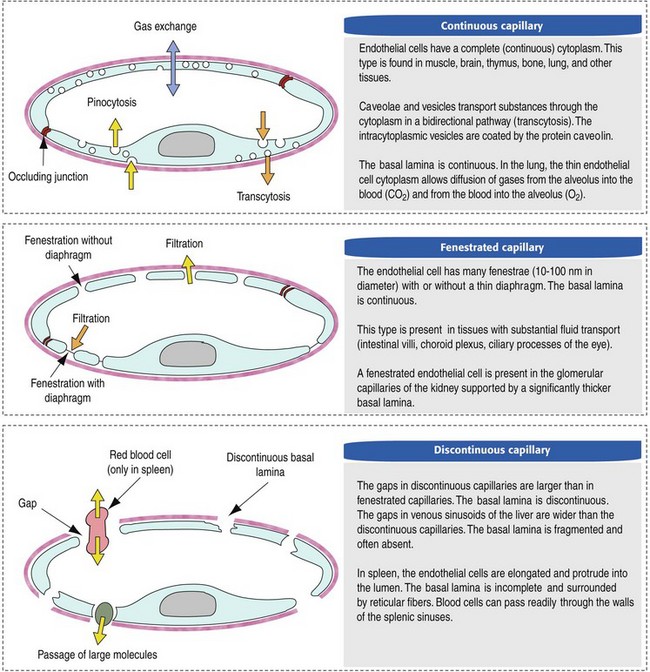
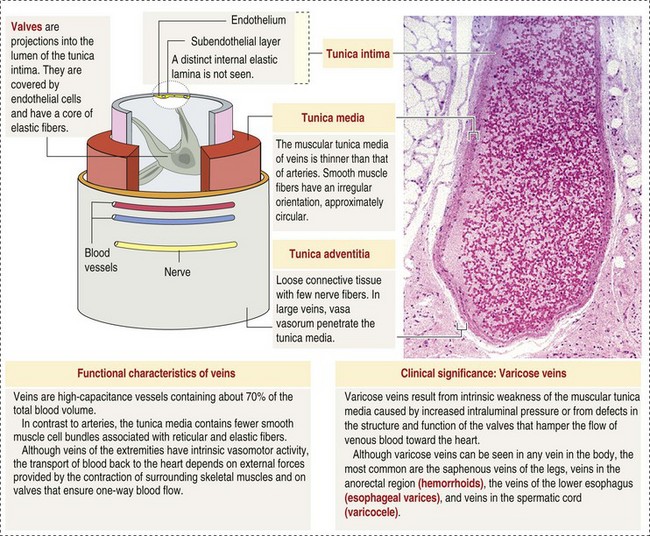
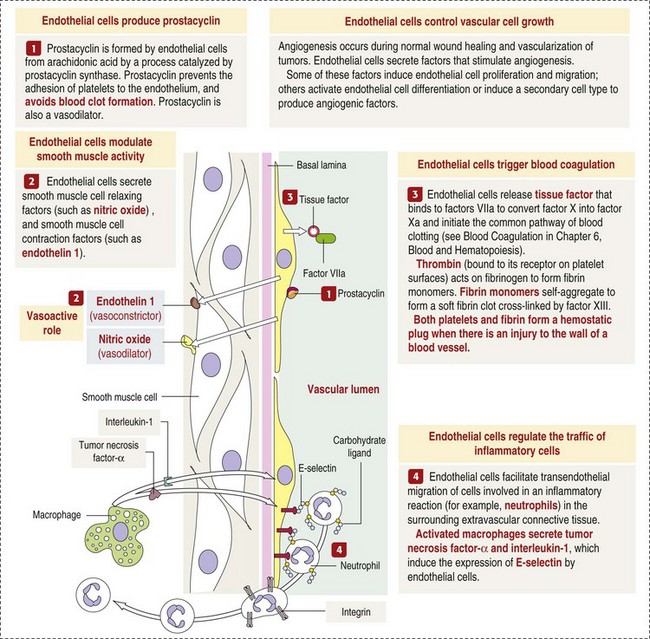
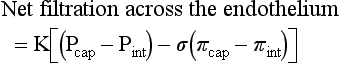CHAPTER 2 The circulatory system consists of blood, a central pump (heart), blood distribution (arterial) and collection (venous) networks, and a system for exchange of nutrients and waste products between blood and extravascular tissue (microcirculation) (Fig. 2-1). A network of lymphatic vessels that parallel the veins also contributes to circulation by draining fluid from extravascular spaces into the blood vascular system. Fig. 2-1 The vascular system. Arteries have relatively large diameter lumens to facilitate rapid blood flow with minimal resistance. Artery walls are thick and consist predominantly of smooth muscle fibers for tensile strength and elastic fibers for elasticity (Web Fig. 2-1). Elastic fibers allow arteries to act as pressure reservoirs, expanding to hold blood ejected from the heart during contraction and passively recoiling to provide continuous flow and pressure to arterioles between heart contractions. Capillaries are the site of nutrient and waste product exchange between the blood and tissue. Capillaries are the most numerous vessel in the circulatory system, with a total cross-sectional area nearly 1300 times that of the aorta. However, they normally contain only about 5% of the total blood volume. The velocity of blood flow through the capillaries is very slow, and red blood cells generally move through a capillary in single file to further facilitate the diffusion of nutrients and wastes. Capillaries have narrow lumens (approximately 8 µm) and thin walls (approximately 1 µm) consisting of a single epithelial cell layer (endothelium). At the junctions between capillary endothelia are interendothelial pores, which make the capillary semipermeable to facilitate diffusion of nutrients and waste products between the blood and tissues. There are three types of capillaries: continuous, fenestrated, and discontinuous. The basic functions and tissue locations of these types of capillaries are illustrated in Fig. 2-2. They are discussed in greater detail in the chapters covering the diseases of organ systems. The return trip of blood to the heart begins in the postcapillary venules. Venules have a composition similar to capillaries but may have thin layers of muscle as they become more distant from the capillary bed. Veins are composed mainly of collagen with smaller amounts of elastin and smooth muscle (Web Fig. 2-2). Venules and veins provide a low resistance pathway for the return of blood to the heart. Because of their distensibility, they can store large amounts of blood; nearly 65% of total blood volume is normally present within the systemic veins. Pressure and velocity of flow are low within venules and veins. Therefore other factors are necessary to help move venous blood toward the heart such as venous valves to prevent backflow of blood, skeletal muscle contraction, venous vasoconstriction, an increased pressure gradient due to decreased pressure in the heart during filling (cardiac-suction effect), and decreased pressure in the thoracic veins due to negative pressure within the thoracic cavity (respiratory pump). The lymphatic system originates as blind-ended lymphatic capillaries, which permeate the tissue surrounding the microcirculation (arterioles, metarterioles, capillaries, and postcapillary venules [Fig. 2-3]).Lymphatic capillaries have overlapping endothelial cells and large interendothelial gaps so that external pressure allows movement of fluid and molecules into the vessel. However, intravascular lymphatic pressure forces these overlapping edges together to prevent the flow of lymph out of the vessel. Lymphatic capillary gaps are much larger than those between blood capillary endothelium, so they can accommodate movement of larger particles and substances. Lymphatic capillaries converge into progressively larger lymph vessels that drain into lymph nodes and then ultimately empty into the venous system. Similar to venous vessels, lymphatics are distensible, low-pressure vessels that require lymphatic valves and contraction of surrounding muscles to facilitate return of fluid to the blood. Fig. 2-3 The microcirculation. All components of the circulatory system are lined by a single layer of endothelium. Endothelium forms a dynamic interface between blood and tissue and is a critical participant in fluid distribution, inflammation, immunity, angiogenesis, and hemostasis (Fig. 2-4). Normal endothelium is antithrombotic and profibrinolytic and helps maintain blood in a fluid state, but when injured, endothelium becomes prothrombotic and antifibrinolytic. Endothelial activation by oxidative stress, hypoxia, inflammation, infectious agents, tissue injury, or similar events results in the production and release of numerous substances with wide-ranging roles in physiology and pathology (Fig. 2-5 and Box 2-1). Endothelial activation is typically localized to restrict a host response to a specific area, while not affecting the normal function of endothelium and flow of blood in other parts of the body. Fig. 2-4 Structure and function of the endothelium. Water distribution between the plasma and interstitium is determined mainly by osmotic and hydrostatic pressure differentials between the compartments and is described by the following formula (Fig. 2-6): Fig. 2-6 Factors affecting fluid balance in the microcirculation. Changes in distribution of fluid between the plasma and interstitium are most commonly manifested as edema, which is an accumulation of excess interstitial fluid. Edema occurs by four major mechanisms: (1) increased microvascular permeability, (2) increased intravascular hydrostatic pressure, (3) decreased intravascular osmotic pressure, and (4) decreased lymphatic drainage (Box 2-2). Edema is morphologically characterized by clear to slightly yellow fluid that generally contains a small amount of protein (transudate), which thickens and expands affected interstitium (Fig. 2-7). When edema occurs in tissues adjacent to body cavities or open spaces, such as alveolar lumens, the increased interstitial pressure often forces fluid into these cavities and spaces. The result can be fluid within alveolar lumens (pulmonary edema; Fig. 2-8), the thoracic cavity (hydrothorax), the pericardial sac (hydropericardium), or the abdominal cavity (ascites or hydroperitoneum; Fig. 2-9). Histologically, edema is an amorphous, pale eosinophilic fluid (hematoxylin and eosin [H&E] stain) because of its protein content (Fig. 2-10). The clinical significance of edema is variable, depending mainly on its location. Subcutaneous edema results in doughy to fluctuant skin and subcutis that is often cooler than adjacent unaffected tissue, but alone has minimal clinical impact (Fig. 2-11). Likewise, ascites does not generally have an impact on the function of abdominal organs. In contrast, edema of a tissue within a confined space, such as the brain in the cranial vault, can result in pressure within the organ that results in serious organ dysfunction. Similarly, filling a confined space with fluid, such as in hydrothorax or hydropericardium, can have a substantial impact on the function of the lungs and heart, respectively. In these situations, edema can have immediate and life-threatening implications. Fig. 2-7 Edema, intestine, submucosa, horse. Fig. 2-8 Pulmonary edema, lung, pig. Fig. 2-9 Ascites (hydroperitoneum), peritoneal cavity, dog. Fig. 2-10 Pulmonary edema, lung, rat. Fig. 2-11 Subcutaneous edema, congenital lymphedema, skin, dog. Normal endothelium provides a surface that promotes the smooth, nonturbulent flow of blood. It produces and responds to mediators that enhance vasodilation, and inhibit platelet activation and coagulation. In contrast, after injury or activation, endothelium produces or responds to mediators that induce vasoconstriction, enhance platelet adhesion and aggregation, and stimulate coagulation (Box 2-3). Platelets are anucleate cell fragments derived from megakaryocytes. Their major role in hemostasis is to form the initial plug that covers and seals a small area of vascular damage. After vascular damage, platelets adhere to subendothelial collagen and other ECM components (e.g., fibronectin, adhesive glycoproteins, and proteoglycans). Adhered platelets express receptors that promote aggregation of additional platelets and become activated to release the products of their cytoplasmic granules and produce other mediators of coagulation (e.g., thromboxane; Box 2-4). The phospholipid surfaces of aggregated platelet membranes also provide a surface to concentrate activated coagulation factors to promote coagulation. The sequence of events that contribute to hemostasis are (1) transient vasoconstriction and platelet aggregation to form a platelet plug at the site of damage (primary hemostasis), (2) coagulation to form a meshwork of fibrin (secondary hemostasis), (3) fibrinolysis to remove the platelet/fibrin plug (thrombus retraction), and (4) tissue repair at the damaged site (Fig. 2-12). Fig. 2-12 Diagrammatic representation of the normal hemostatic process. Primary hemostasis includes the initial vascular and platelet response to injury. Neurogenic stimuli and mediators released locally by endothelium and platelets cause vasoconstriction immediately after damage (Fig. 2-12, A). The nature and effectiveness of vasoconstriction is partially determined by the size of the affected vessel, the amount of smooth muscle it contains, and endothelial integrity. Narrowing of the vessel lumen allows opposing endothelial surfaces to come into contact with and sometimes adhere to each other to reduce the volume of blood flowing through the damaged area. Platelets can directly adhere to the exposed subendothelial matrix of collagen, fibronectin, and other glycoproteins and proteoglycans (Fig. 2-12, B). However, more efficient adhesion occurs when von Willebrand’s factor released by local activated endothelium coats subendothelial collagen to form a specific bridge between collagen and the glycoprotein platelet receptor GPIb. At this stage and without further stimulation, adhered and aggregated platelets may disaggregate. Otherwise, platelets within the aggregate secrete the contents of their dense bodies and α-granules and produce substances such as thromboxane to accelerate hemostasis. Adenosine diphosphate (ADP) released from dense granules triggers the binding of fibrinogen to platelet receptor GPIIb-IIIa, resulting in the formation of fibrinogen bridges that link platelets into a loose aggregate. Platelet contraction consolidates this loose aggregate into a dense plug, which covers the damaged area. When vascular injury is minimal, platelet plugs alone may resolve the damage. If not, the exposed collagen and aggregated platelet phospholipids promote secondary hemostasis at the site.
Vascular Disorders and Thrombosis
Circulatory System
Blood travels from the left to the right side of the heart via the systemic circulation, and from the right to the left side via the pulmonary circulation. Blood flow rate and pressure in the systemic arterial circulation decrease in conjunction with increased arterial cross-sectional area. In the venous systemic circulation, blood flow rate, but not pressure, increases in conjunction with decreased venous cross-sectional area. The flow, pressure, and cross-sectional area relationships are similar but reversed (i.e., veins deliver blood and arteries collect blood) in the pulmonary circulation. (Courtesy Dr. D.A. Mosier and L. Schooley, College of Veterinary Medicine, Kansas State University.)
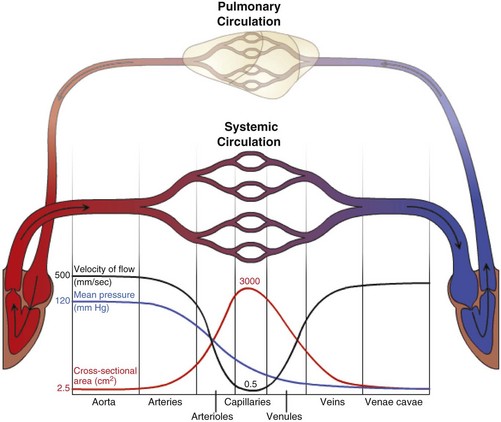
The microcirculation consists of arterioles (small arteries proximal to a capillary bed), metarterioles (arterial capillaries), capillaries (thin, semipermeable vessels that connect arterioles and venules), and postcapillary venules (small vessels that merge to form veins after collecting blood from a capillary network). Smooth muscle of the arterioles and metarterioles regulates flow of blood into the capillary bed. There is a dramatic drop in pressure and flow rate from the arterial to the venous side of the microcirculation, facilitating interactions between capillary blood and interstitial fluid. Blind-ended lymphatic vessels that originate near capillary beds interact intimately with the microcirculation. (Courtesy Dr. D.A. Mosier and L. Schooley, College of Veterinary Medicine, Kansas State University.)
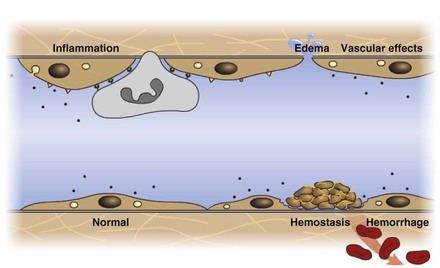
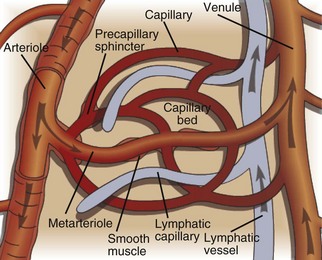
Endothelium is a physical barrier between intravascular and extravascular spaces, and it is an important mediator of fluid distribution, hemostasis, inflammation, and healing. (Courtesy Dr. D.A. Mosier and L. Schooley, College of Veterinary Medicine, Kansas State University.)
Fluid Distribution and Homeostasis
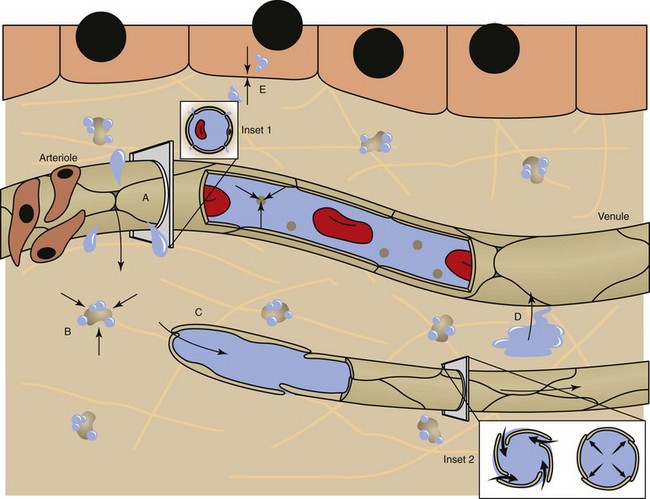
Fluid distribution is determined by physical characteristics of the microcirculation and lymphatics and osmotic and hydrostatic forces within the blood and interstitial fluid. Intercellular gaps between endothelium allow movement of fluid and small molecules between the blood and interstitial fluid (insets 1 and 2). A, High arteriolar hydrostatic pressure forces fluid into the interstitium. B, Plasma proteins (e.g., albumin) and molecules within the ECM exert an osmotic effect to attract and retain water. C, Interstitial hydrostatic pressure forces interstitial fluid into lower pressure venules. D, The slight excess of interstitial fluid not returned to the venules enters the lymphatics to be drained from the area. E, Exchange of intracellular and interstitial fluid is balanced by osmotic forces and concentration gradients of electrolytes and other molecules across the cell plasma membrane. Inset 1, Cross-section of a blood vessel capillary showing interendothelial junctions. Endothelium forms end-to-end junctions for movement of fluid and small molecules. Inset 2, Cross-section of a lymphatic capillary showing the interendothelial junctions. Endothelium overlaps to allow movement of larger particles and closure when intravascular pressure forces overlapping endothelium together. (Courtesy Dr. D.A. Mosier and L. Schooley, College of Veterinary Medicine, Kansas State University.)
Abnormal Fluid Distribution
Imbalance Between Intravascular and Interstitial Compartments (EDEMA)
Mechanisms of Edema Formation
Morphologic Characteristics of Edema
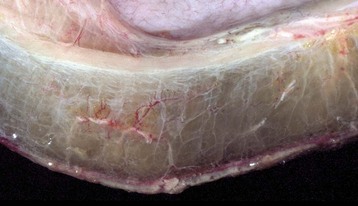
Note the clear to slightly yellow fluid (that generally contains a small amount of protein [transudate]), which thickens and expands the affected submucosa. (Courtesy Department of Veterinary Biosciences, The Ohio State University; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
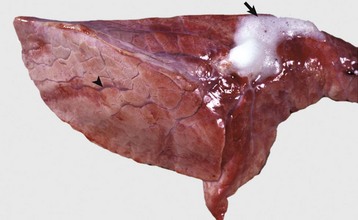
The lung failed to collapse and is heavy and firm due to edema fluid in alveoli and the interstitium. Note the prominent interlobular septa caused by edema (arrowhead) and the frothy edema fluid exuding from the bronchus (arrow). (Courtesy Dr. M.D. McGavin, College of Veterinary Medicine, University of Tennessee.)
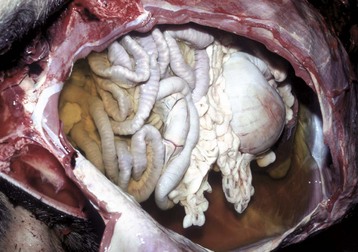
Slightly yellow fluid is present in the peritoneal cavity. When edema occurs in tissue adjacent to body cavities, the increased interstitial pressure forces the edema fluid, which is usually clear to slightly yellow (transudate), into these cavities. (Courtesy Dr. D.A. Mosier, College of Veterinary Medicine, Kansas State University.)
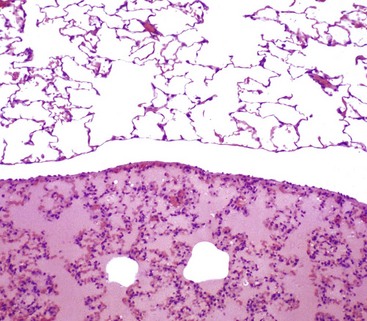
There is eosinophilic (pink staining) fluid distending the alveoli in the lower specimen. Histologically, edema is an amorphous, pale eosinophilic fluid, and the depth of the eosinophilia is proportional to its protein content. The fluid in this specimen has a high protein content. The upper specimen is normal rat lung. H&E stain. (Courtesy Dr. A. López, Atlantic Veterinary College; and Noah’s Arkive, College of Veterinary Medicine, The University of Georgia.)
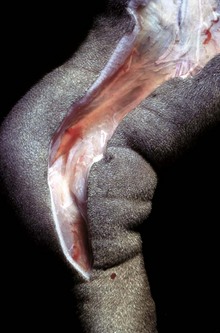
This form of edema results in doughy to fluctuant skin and subcutis. Edematous skin is often cooler than adjacent unaffected skin. In congenital lymphedema, the lymph vessels are hypoplastic or aplastic. (Courtesy Dr. H. Liepold, College of Veterinary Medicine, Kansas State University.)
Hemostasis
Hemostatic Process
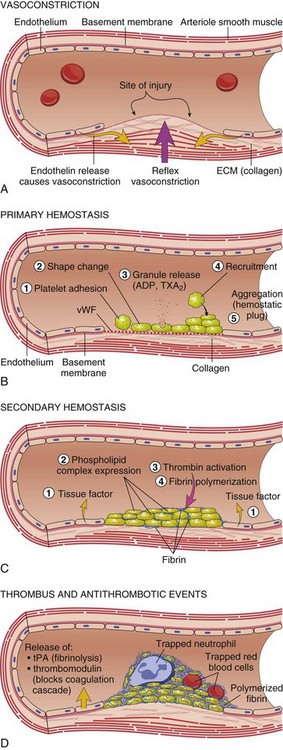
A, After vascular injury, local neurohumoral factors induce a transient vasoconstriction. B, After endothelial injury and disruption that exposes the subendothelial extracellular matrix (ECM), platelets adhere to the ECM via von Willebrand’s factor (vWF) and are activated, undergoing a shape change and granule release; released adenosine diphosphate (ADP) and thromboxane A2 (TXA2) lead to further platelet aggregation to form the primary hemostatic plug. C, Local activation of the coagulation cascade (involving tissue factor and platelet phospholipids) results in fibrin polymerization, “cementing” the platelets into a definitive secondary hemostatic plug. D, Counter-regulatory mechanisms, such as release of tissue plasminogen activator (tPA) (fibrinolytic) and thrombomodulin (interfering with the coagulation cascade), limit the hemostatic process to the site of injury. (From Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Primary Hemostasis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Vascular Disorders and Thrombosis

 is intracellular and
is intracellular and 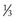 is extracellular (80% of which is in the interstitium and 20% is in the plasma). The distribution of fluid-nutrients, and waste products between the blood, interstitium, and cells is controlled by physical barriers, as well as pressure and concentration gradients between each compartment. The cell’s plasma membrane is a selective barrier that separates interstitial and intracellular compartments. Nonpolar (uncharged) lipid-soluble substances, such as O2, CO2, and fatty acids, move relatively freely across the plasma membrane based on concentration gradients. Polar (charged) lipid-insoluble particles and molecules, such as electrolytes, calcium, glucose, and amino acids, enter the cell by carrier-mediated transport. Water readily moves across the plasma membrane down its concentration gradient. Although approximately 100 times the volume of water in a cell crosses the plasma membrane in 1 second, cell fluid content remains relatively stable because of the activity of energy-dependent membrane pumps (e.g., Na+/K+-adenosine triphosphatase [ATPase] pump) and the balance between osmotic pressures exerted by interstitial and intracellular solutes.
is extracellular (80% of which is in the interstitium and 20% is in the plasma). The distribution of fluid-nutrients, and waste products between the blood, interstitium, and cells is controlled by physical barriers, as well as pressure and concentration gradients between each compartment. The cell’s plasma membrane is a selective barrier that separates interstitial and intracellular compartments. Nonpolar (uncharged) lipid-soluble substances, such as O2, CO2, and fatty acids, move relatively freely across the plasma membrane based on concentration gradients. Polar (charged) lipid-insoluble particles and molecules, such as electrolytes, calcium, glucose, and amino acids, enter the cell by carrier-mediated transport. Water readily moves across the plasma membrane down its concentration gradient. Although approximately 100 times the volume of water in a cell crosses the plasma membrane in 1 second, cell fluid content remains relatively stable because of the activity of energy-dependent membrane pumps (e.g., Na+/K+-adenosine triphosphatase [ATPase] pump) and the balance between osmotic pressures exerted by interstitial and intracellular solutes.