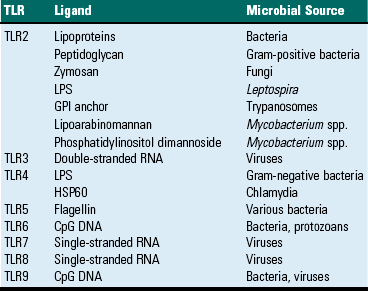
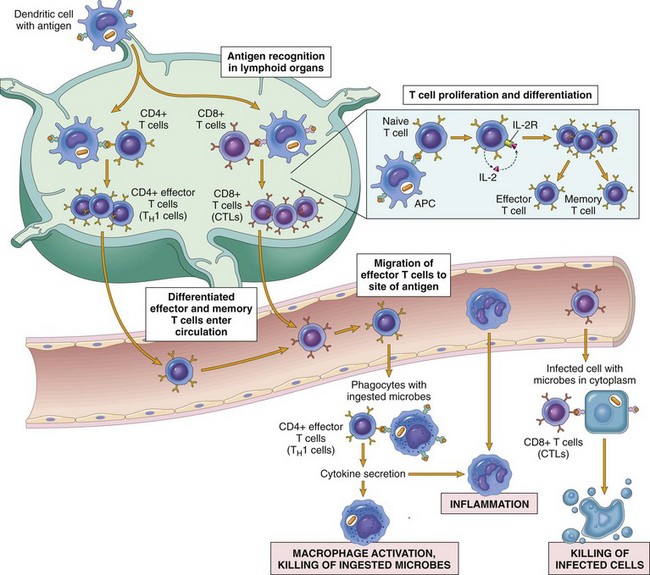
CHAPTER 5 As stated previously, the function of the immune system is to protect against infectious pathogens and the development of cancer. There are two categories of immune responses that are based in part on their specificity for the antigen: (1) innate immunity and (2) adaptive immunity (Fig. 5-1). Innate immune responses are considered the first-line defense mechanisms, are not specific to the antigen, and lack memory. These defense mechanisms are the result of anatomic (e.g., skin, mucosal epithelia, cilia) and physiologic (e.g., stomach pH, body temperature) properties, and phagocytic and inflammatory responses. Major components of innate immunity are intact epithelial barriers, phagocytic cells, natural killer (NK) cells, and a number of plasma proteins, the most important of which are the proteins of the complement system. Phagocytic cells are recruited to sites of infection during an inflammatory response where they have a number of functions, two of which are to ingest and destroy pathogenic organisms and neutralize toxins. Neutrophils, monocytes, and tissue macrophages are the major cells involved in phagocytosis. These cells recognize components of microbial pathogens through the expression of several membrane receptors, including receptors for mannose residues and N-formyl-methionine containing peptides and a family of pattern recognition receptors (PRRs) that, when activated by microbial components, signal the activation of transcription factors that facilitate the microbicidal mechanisms of the phagocytic cell and are discussed later. NK cells are the cytotoxic cells of innate immunity and are also discussed later. The complement system, discussed in Chapter 3, is a complex cascade of proteins that has a number of biologic functions, including the formation of the membrane attack complex that efficiently lyses plasma membranes of microbial pathogens. The complement system can be activated by either the innate immune system (alternative and mannose/lectin pathways) or the adaptive immune system (classical pathway). Other important plasma proteins of the innate immune system include mannose-binding protein and C-reactive protein; two of the functions of these proteins are to facilitate phagocytosis through opsonization of pathogens and complement activation. Inflammatory responses comprise vascular, permeability, and cellular phases that act in response to damage to vascularized tissue. The features of the inflammatory response are also presented in Chapter 3. Fig. 5-1 Innate (nonspecific) and adaptive (specific) immunity are depicted in relation to the time course of an infection. Members of the NLR are central regulators of immunity and inflammation largely through activation of transcription factors like nuclear factor (NF) kappa B, interferon regulatory factor (IRF), or nuclear factor of activated T lymphocytes (NFAT). Some members of the NLR family form multiprotein complexes with the cysteine protease pro-caspase-1 and the adapter molecule ASC (apoptosis-associated speck-like protein containing a CARD) referred to as inflammasomes (also see Fig. 3-12). The inflammasome is a multiprotein complex that activates caspase-1. PAMPs and DAMPs are sensed through activation of the inflammasome complex, resulting in activation of caspase-1, which elicits effector functions through proteolytic cleavage of cytosolic proinflammatory cytokines (e.g., pro-interleukin [IL]-1β and pro-IL-18), which are then secreted in their active form. The inflammasome model is analogous to the model for the activation of the apoptotic caspases by CD95/Fas death–inducing signaling complex (DISC) and the Apaf-1 apoptosome. IL-1β functions in localized and systemic responses to infections and injury, to cause fever, activation of lymphocytes, and the extravasation of leukocytes at sites of injury or infection. IL-18 functions to induce IFN-γ by activated T lymphocytes and NK cells during a TH1 response and to induce secondary inflammatory cytokines, chemokines, cell adhesion molecules, and nitric oxide (NO) synthesis. Other members of the NLR family are involved in inflammasome-independent (noninflammasome) innate immune responses and signal through different multicomponent signalosomes such as nodosomes, transcriptosomes, mito-signalosomes. Noninflammasome-mediated innate immune responses occur through NF kappa B activation, mitogen-activated protein kinase (MAPK) activation, cytokine and chemokine production, antimicrobial reactive oxygen species production, type I interferon (IFN-α and IFN-β) production, and ribonuclease L activity. TLRs are the mammalian homologue of the Toll receptor originally identified in Drosophila. It has not only an embryologic function but also an immunologic function. In mammals, TLRs are membrane molecules that function in cellular activation by a wide range of microbial pathogens. TLRs are classified as PRRs because they recognize PAMPs and signal to the host the presence of an infection. Pathogen associated molecules include lipopolysaccharide (LPS) from Gram-negative bacteria, peptidoglycan from Gram-positive bacteria, double-stranded RNA from viruses, or α-glucans from fungi (Table 5-1). In general, TLRs 1, 2, 4, and 6 recognize unique bacterial products that are found on the cell surface, and TLRs 3, 7, 8, and 9 are involved in viral detection and nucleic acid recognition within endosomes. The specificity of TLRs for microbial products depends on interactions between TLRs and non-TLR adapter molecules. All TLRs contain an extracellular domain characterized by a leucine-rich repeat motif flanked by a cysteine-rich motif (Fig. 5-2). They also contain a conserved intracellular signaling domain, Toll/IL-1 receptor (TIR), that is identical to the cytoplasmic domain of the IL-1 and IL-18 receptors. Fig. 5-2 illustrates how TLRs function in the recognition of LPS. In the blood or extracellular fluid, the binding of LPS to LPS-binding protein (LBP) facilitates the binding of LPS to CD14, a plasma protein and glycophosphatidylinositol-linked membrane protein present on most cells. The binding of LPS to CD14 results in the dissociation of LBP and the association of the LPS-CD14 complex with TLR4. An accessory protein, MD2, complexes with the LPS-CD14-TLR4 molecule and results in LPS-induced cell signaling. TABLE 5-1 Toll-Like Receptors (TLRs) and TLR Ligands and Their Microbial Source Modified from Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders. Fig. 5-2 Signaling pathway for Toll-like receptor 4 (TLR4) in response to bacterial lipopolysaccharide (LPS). Adaptive immunity in general consists of cell-mediated immunity, mediated by T lymphocytes against intracellular pathogens, and humoral immunity, mediated by B lymphocytes against extracellular pathogens and toxins (Fig. 5-3). The adaptive immune response is the second-line defense mechanism and is characterized by antigen specificity, diversity, memory, and self-/nonself-recognition. Antigen specificity and self-/nonself-recognition are the result of distinct membrane molecules. Mature B lymphocytes are activated by a specific antigen-binding molecule on its membrane. The antigen receptor is membrane-bound immunoglobulin. Mature T lymphocytes express a specific antigen-binding molecule, the T lymphocyte receptor (TCR), on their membrane. Unlike membrane-bound immunoglobulin on the B lymphocyte, which can recognize antigen alone, TCRs can recognize only antigens that are associated with cell membrane proteins called MHC molecules. Self-/nonself-recognition is the result of MHC molecules. There are two major classes of MHC molecules. Class I molecules are present on all nucleated cells, and class II molecules are present primarily on antigen-presenting cells. T lymphocytes and B lymphocytes are the major cells of adaptive immunity. Fig. 5-3 Overview of humoral and cell-mediated arms of adaptive immunity. T lymphocytes are small nongranular cells that constitute 50% to 70% of the peripheral blood mononuclear cells. They originate in the bone marrow and migrate to the thymus (thus the “T” designation), where they undergo differentiation, selection, and maturation processes before exiting to the periphery as effector lymphocytes. In secondary lymphoid tissues, they are located primarily in the paracortical regions of lymph nodes and the periarteriolar sheaths (PALS) of the spleen. These specific anatomic sites elaborate chemoattractant cytokines (chemokines), for which the T lymphocytes express receptors. The definitive marker for T lymphocytes is the TCR, the polymorphic antigen-binding molecule. The antigen specificity of individual lymphocytes is attributed to their respective TCR, which is genetically determined. TCRs are classified as either αβ-TCR or γδ-TCR based on the composition of their disulfide-linked heterodimers. The individual polypeptide chains of the heterodimers contain variable (antigen-binding) and constant regions. In mammals, most peripheral blood T lymphocytes express αβ-TCR; however, in ruminants and swine, these lymphocytes make up only 10% to 50%, and 10% of peripheral blood T lymphocytes, respectively. Both TCRs are associated with CD3, and together they form the TCR-CD3 complex. There are significant activation and functional differences between αβ-TCR– and γδ-TCR–expressing lymphocytes. Unlike membrane-bound immunoglobulin on B lymphocytes that can recognize soluble antigen, the αβ-TCR can only recognize antigen after it has been processed into peptide fragments and associated with MHC molecules (the MHC is discussed later). In most instances, the antigen is associated with the MHC on the surface of an antigen-presenting cell, a virally infected cell, a neoplastic cell, or a cell of a foreign tissue graft. Individual αβ-TCRs are covalently linked to a cluster of five polypeptide chains, three comprising the CD3 molecule and two comprising the β-chain. The CD3 molecule and the β-chain are invariant, and although they do not bind antigen, they do function in the signal transduction after antigen binding by the TCR. Each T lymphocyte expresses a unique TCR with regard to structure and antigen specificity. The genes that encode α-, β-, γ-, and δ-chains of the TCR can undergo somatic rearrangements during their development in the thymus, resulting in tremendous diversity for antigen recognition. Not only are these rearrangements important for diversity, but also they can be used to molecularly phenotype proliferating populations of T lymphocytes as a diagnostic tool for the identification of clonal populations (neoplastic) and polyclonal populations (nonneoplastic) (see Chapter 6). Although all T lymphocytes express the TCR-CD3 complex, they are further classified according to accessory CD4 and CD8 molecules. These nonpolymorphic accessory molecules include CD4, CD8, CD2, integrins, and CD28. CD4 and CD8 functionally subdivide T lymphocytes into CD8+ cytotoxic T lymphocytes (CTL [TC]) and CD4+ helper T lymphocytes (TH). During antigen presentation, CD4+ lymphocytes only recognize antigen bound to MHC class II molecules (Fig. 5-4), whereas CD8+ lymphocytes only recognize antigen bound to MHC class I molecules. This coreceptor requirement is commonly referred to as MHC class I and MHC class II restriction, the basis for positive selection in the thymus. Although there have been reports in some species of CD4 lymphocytes that are functionally cytotoxic and CD8 lymphocytes that are functionally “helper”-like, these appear to be anomalies and for the purposes of this text are excluded. In most species, peripheral blood T lymphocytes express either CD4 or CD8. Except for ruminants and swine, lymphocytes negative for both CD4 and CD8—“double negative” lymphocytes—are rare in the peripheral blood. So-called “double positive” lymphocytes, positive for both CD4 and CD8, are rare, except in swine, where they can approach 25% of the T lymphocytes in the peripheral circulation. T lymphocytes require two signals for activation. Signal 1 is provided by the TCR and the MHC-antigen complex and the CD4 or CD8 MHC complex. Signal 2 is provided by another accessory molecule expressed by T lymphocytes, the CD28 molecule. The ligands for CD28 are B7-1 (CD80) and B7-2 (CD81) expressed on activated dendritic cells, B lymphocytes, and macrophages (see Fig. 5-4). An inability to deliver the second signal results in an unresponsive T lymphocyte that either undergoes apoptosis or remains anergic. These molecules provide an important co-stimulatory signal for T lymphocyte activation, and are discussed in more detail later in the chapter regarding anergy and the development of tolerance with regard to autoimmunity. When T lymphocytes are activated by antigen and receive the appropriate co-stimulatory signals, they clonally expand as a result of their secretion of IL-2. This clonally expanded population of T lymphocytes, of the same antigen specificity, differentiates into populations of effector lymphocytes and memory lymphocytes. Fig. 5-4 The T lymphocyte receptor complex (T lymphocyte receptor [TCR]). TH lymphocytes can be classified based on their functional capacity and ability to elicit primarily an antibody response or a cell-mediated immune response. After activation of TH lymphocytes, by recognition of antigen bound to MHC class II molecules on the surface of an antigen-presenting cell, there is clonal expansion of TH lymphocytes of the same antigen specificity. These clonally expanded lymphocytes are important in directing the immune response as either primarily an antibody response or a cellular response. The type of response is dictated by a restricted cytokine profile that primarily activates B lymphocytes in the case of an antibody response or activates CTL (Tc) and macrophages in a cellular response. The restricted cytokine profile for TH lymphocytes allows for their classification as either TH1 or TH2 lymphocytes (Web Table 5-1). TH1 lymphocytes synthesize and secrete IL-2 and interferon-γ (IFN-γ), stimulating CTL (Tc) and macrophages, and induce a cell-mediated immune response. TH2 lymphocytes synthesize and secrete IL-4, IL-5, IL-6, and IL-13 that stimulate B lymphocytes to develop into antibody-secreting plasma cells and inhibit macrophage functions, and induce an antibody response. The type of immune response (antibody versus cell-mediated) can have a profound influence on the outcome of a disease. In the instance of an intracellular protozoal infection, a TH2 type response results in rapid proliferation of the organism and death of the host, whereas a TH1 type response results in elimination of the organism and survival of the host. Similarly, a TH2 response to an allergen results in the elaboration of immunoglobulin (Ig) E, through IL-4 production, stimulation of eosinophils, through IL-5 production, and the development of an allergic reaction. The exact regulation of the TH1 versus the TH2 lymphocyte response is unknown, but studies suggest that IL-12 produced by activated macrophages stimulates the TH1 response, whereas IL-4 inhibits the TH1 response, allowing the TH2 response to dominate. TH lymphocytes predominately drive the immune response to microbial pathogens by activating macrophages or B lymphocytes. Another functionally distinct subpopulation of CD4+ T lymphocytes is the regulatory T lymphocyte (T reg). T reg lymphocytes function to suppress the response of self-reactive CD4 lymphocytes that have escaped the negative selection process in the thymus. They are distinguished from other CD4 T lymphocytes by the expresson of CD25 on the cell surface. Like TH1 and TH2 lymphocytes, T reg lymphocyte differentiation is driven by cytokine environments; however, where TH1 and TH2 lymphocyte activation occurs through transcripional activators T-bet and GATA-3, respectively, T reg lymphocytes are activated through the transcriptional repressor FoxP3 (Fig. 5-5). This subpopulation of T reg lymphocytes are often referred to as Fox3P lymphocytes and produce the immunosuppressive and antiinflammatory cytokines IL-4, IL-10 and TGF-β. FoxP3 lymphocytes are an intense area of investigation for their role as suppressor lymphocytes of immunity and inflammation. T reg lymphocytes have been shown to have a role in the prevention of organ-specific autoimmune diseases and in modulation of immune responses to microbial pathogens to prevent overwhelming inflammatory reactions. Finally, a subpopulation of CD4 lymphocytes characterized by the ability to produce IL-17 is designated as TH17 lymphocytes. TH17 lymphocyte differentiation is driven by TGF-β, IL-6, IL-1, and IL-23. TH17 lymphocytes, through production of chemokines IL-17 and IL-22, induce the recruitment of monocytes and neutrophils to sites of inflammation. Again, one must recognize that this is an oversimplification of a complex regulatory mechanism and that as additional knowledge is gained about TH1, TH2, T reg, and TH17 lymphocyte responses, we will be able to understand pathogenic mechanisms of diseases, which will lead to the development of more specific therapeutic targets. Fig. 5-5 Differentiation and expression of CD4 T lymphocytes. WEB TABLE 5-1 Basic Cytokine and Functional Profiles of TH1 and TH2 Lymphocytes B lymphocytes constitute 5% to 20% of the peripheral blood mononuclear cells. B lymphocyte development occurs in two phases, an antigen-independent phase in the primary lymphoid tissues, followed by an antigen-dependent phase in secondary lymphoid tissues. B lymphocytes can be found in primary lymphoid tissues, such as the bone marrow and ileal Peyer’s patches (a primary lymphoid tissue in some species because it is the site of B lymphocyte development, rather than the bone marrow), and in secondary lymphoid tissues, such as the spleen, lymph nodes, tonsils, and Peyer’s patches. Within secondary lymphoid tissues, B lymphocytes are aggregated in the form of distinct lymphoid follicles, which on activation expand to form prominent pale regions called germinal centers (Fig. 5-6). This anatomic localization, similar to T lymphocytes in the PALS and paracortex, is the result of elaboration of chemokines for which the B lymphocyte has receptors. The antigen receptor of the B lymphocyte is the membrane bound immunoglobulin. After the antigen-independent phase of development, B lymphocytes express IgM and IgD on their surface that signifies a mature B lymphocyte. In the antigen-dependent phase, antigen-activated mature B lymphocytes differentiate into IgM-secreting plasma cells or switch to another antibody isotype. Immunoglobulins can be generated against an almost unlimited number of antigenic determinants through the rearrangement of genes encoding the light chain and heavy chain components. As in the case of the TCR, an evaluation of the rearranged genes of a B lymphocyte can be used to molecularly phenotype B lymphocyte neoplasms (see Chapter 6). Fig. 5-6 Histology of a hyperplastic lymph node. Like the T lymphocyte, the B lymphocyte also has accessory molecules that function to form the antigen receptor complex (Fig. 5-7). These nonpolymorphic molecules are nonpolymorphic heterodimers composed of Ig-α (CD79a) and Ig-β (CD79b) that do not bind antigen but do interact with the transmembrane portion of surface immunoglobulin involved in cell activation. B lymphocytes, unlike T lymphocytes, can recognize soluble antigens. Additional nonpolymorphic molecules that are important to B lymphocyte functions are CD21 and CD40. The CD21 molecule is the complement receptor-2 molecule whose ligands are C3b and C3d. B lymphocyte responses to protein antigens are dependent on cytokines produced by activated T lymphocytes (CD4+). The CD40 molecule interacts with CD40 ligand on the surface of TH lymphocytes and functions to allow B lymphocyte development into antibody-secreting plasma cells. A failure to express CD40 ligand has been associated with an inability to isotype switch, resulting in a hyper-IgM syndrome. B lymphocytes activated by antigen develop into antibody secreting plasma cells and memory lymphocytes of the same antigenic specificity. Fig. 5-7 The B lymphocyte antigen receptor complex. In general, a circulating MPS cell in the blood is designated as a monocyte, whereas the tissue-based cell is designated as a macrophage. The blood monocyte-to-tissue macrophage is well recognized, although the mechanisms that allow for differentiation of the circulating monocyte pool into the tissue-based pool are still largely unknown. The monocyte is a bone marrow–derived cell of the myeloid lineage that is the precursor cell to the terminally differentiated macrophage that has limited recirculation and replication capacity. The myeloid dendritic cell represents a specific type of mononuclear cell present in nonlymphoid tissues with unique migratory properties and is discussed separately. As opposed to granulocytic myeloid cells, macrophages are long-lived (days to months) and can exist as quiescent “resident” cells widely distributed throughout the body. It is becoming increasingly clear that there is heterogeneity in the circulating monocyte pool that corresponds with the ultimate tissue localization of resident macrophages. The phenotypic characterization of the cells the MPS contains is often used in an attempt to identify specific populations of MPS (Fig. 5-8). Morphologically, monocytes are variably sized with an irregular shape, oval or kidney-shaped nucleus, prominent cytoplasmic vesicles, and a high cytoplasm to nucleus ratio. These features are not unique to monocytes, and as a result, they are difficult to distinguish from circulating dendritic cells, activated lymphocytes, and NK cells based on morphology or by light scatter (flow cytometry). This section covers basic concepts attributable to monocytes, tissue macrophages, and myeloid dendritic cells and refers to specific organ systems. See additional chapters in the section on pathology of organ systems for details on organ-specific cells of the MPS. Certain organ systems have specific names for their resident macrophages, whereas other organ systems only refer to them as macrophages (Table 5-2). TABLE 5-2 Nomenclature and Location of Nonlymphoid Monocyte-Macrophage Cell Types Growth and differentiation of monocytes is regulated by specific growth factors, such as IL-3, colony-stimulating-factor-1 (CSF-1), granulocyte-macrophage CSF (GM-CSF), IL-4, and IL-13, and inhibitors such as type I interferons and transforming growth factor-β (TGF-β). CSF-1 is the most important because it controls the proliferation, differentiation, and survival of the monocyte. Monocytes represent approximately 4% to 10% of blood leukocytes and are identified in mammals, birds, amphibians, and fish. They are largely viewed as accessory cells that importantly link inflammation and innate immune responses to adaptive immunity. Hematopoietic stem cells (HSC) give rise to the common myeloid progenitor (CMP), the precursor cell to the granulocyte/macrophage progenitor (GMP) and macrophage/dendritic cell progenitor (MDP). The MDP is the common progenitor cell for monocytes, macrophages, and conventional dendritic cells. Monocytes express the CSF-1 receptor (CD115) and the chemokine receptor CX3CR1, are differentiated from polymorphonuclear cells (PMNs), NK cells, and lymphoid cells, and do not express CD3, CD19, or CD15. Monocyte heterogeneity based on surface marker expression and function has identified subsets that are an area of intense investigation and are best characterized in humans and rodents. Subpopulations of blood monocytes can be phenotyped based on the expression of surface markers CD14 and CD 16 (FcγR-III). Two additional subpopulations of blood monocytic cells are the myeloid blood dendritic cells, which are negative for CD14 and CD16. In the mouse, the main subset of CD115+ monocytes are characterized as large cells expressing Ly6C, the chemokine receptor CCR2 and the adhesion molecule L-selectin (CD62L), and CX3CR1 (Fig. 5-9). These are referred to as inflammatory monocytes or inflammatory-monocyte-derived macrophages that are preferentially recruited to inflamed tissues and lymph nodes where they produce high levels of TNF-α and IL-1. The emigration of Ly6C+ monocytes from the bone marrow to the periphery depends on the chemokine receptor CCR2 and its ligands CCL7 and CCL2. Ly6C negative monocytes have less of an influence on inflammatory reactions and appear to function more importantly as tissue resident cells and as cells involved in healing and regeneration associated with vascular injury. In those species characterized to date, there appear to be two major functional subpopulations of monocytes, one that is recruited and differentiated into macrophages at the site of inflammation and expresses higher levels of MHC class II and adhesion molecules and one that is responsible for repopulating resident tissue macrophages. Both populations can give rise to dendritic cells (see Fig. 5-8). Classification schemes are forever evolving, and there are definite species differences that may explain species differences in rates of infection and types of clinical disease associated with specific microbial agents. Similar phenotypic and functional heterogeneity exists with tissue-based macrophages. The MPS of hematopoietic and lymphoid organs is diverse with functionally and phenotypically distinct parallel subpopulations in humans, rodents, and pigs. In primary lymphoid organs, mature macrophages are involved in the production, differentiation, and destruction of all lineages of hematopoietic cells in the bone marrow, and in positive and negative selection processes in the thymus. In secondary lymphoid organs, macrophages are uniquely positioned to enhance their phagocytic properties to endogenous and exogenous material and to influence other cell types through their products. The resident macrophages in the spleen differ in microscopic localization, phenotype, and function. The reason for this complexity is largely attributable the spleen’s role as a hematopoietic organ and as a secondary lymphoid organ. Distinct subpopulations of macrophages are present in the red pulp, white pulp, and marginal zone with some recognized species differences (see Chapter 13). The red pulp macrophage functions in the phagocytosis of hematogenous pathogens and senescent erythrocytes. Erythrophagocytosis primarily occurs in the spleen and liver and allows for the removal of senescent erythrocytes and recycling of iron, often evident as cytoplasmic accumulations of pigments. The white pulp macrophages are also actively phagocytic, and a morphologically distinct cell, the “tingible body macrophage,” can often be easily identified histologically and represents macrophages involved in the uptake and removal of apoptotic T and B lymphocytes. The marginal zone of the spleen provides a complex environment for the interface for the red and white pulp, specifically an important transit area for cells leaving circulation and entering the white pulp, and for B lymphocyte differentiation. The development of granulomatous inflammatory reactions in the spleen as a response to hematogenous microorganisms often begins in the marginal zone. The two subpopulations of macrophages present in the marginal zone are the metallophilic macrophage and the marginal zone macrophage. Marginal zone macrophages express high levels of PRRs and scavenger receptors that facilitate the clearance of pathogens from the circulation. The function of metallophilic macrophages is unknown. Tissue macrophages are derived from a combination of precursors in the blood (monocytes) and from local proliferation of precursors that varies for individual organ systems. Mononuclear phagocytic cells include circulating monocytes and tissue-based macrophages. In the spleen, macrophages are located in the marginal zone, white pulp, and red pulp, where they function primarily as phagocytic cells. In the lymph node, macrophages are located in the subcapsular sinus, which is analogous to the marginal zone of the spleen, and the medulla. These physical locations, the subcapsular sinus of lymph nodes and marginal zone of the spleen, facilitate their exposure to potential antigens. Nonlymphoid tissue-based macrophages have different functions and are named according to the tissue in which they reside (see Table 5-2). One primary function of these cells is phagocytosis, as discussed in Chapter 3. Macrophages express Fc receptors (FcR) for antibody and can phagocytose antigens opsonized by antibody or complement components. Another primary function is their involvement in the immune response as antigen-presenting cells. In this instance, they phagocytose antigen and process it into peptide fragments, which are then presented to T lymphocytes, and the induction of cell-mediated immune responses. Although all nucleated cells express MHC class I molecules and could be considered antigen-presenting cells, only three cell types normally express MHC class II molecules and are regarded as the major antigen-presenting cells. They are the macrophage, dendritic cell, and B lymphocyte. Whereas B lymphocytes and dendritic cells constitutively express MHC class II molecules, macrophages express MHC class molecules on activation. Dendritic cells comprise a distinct population of cells that are characterized by elongate cell processes. Most dendritic cells are antigen-presenting cells, which process antigens and present fragments to T lymphocytes. They are more efficient than macrophages and B lymphocytes at antigen presentation. Antigen-presenting dendritic cells are nonphagocytic, bone marrow–derived cells. They are the most important antigen-presenting cell for initiating primary immune responses to protein antigens (Fig. 5-10). Antigen-presenting dendritic cells express a number of molecules, such as TLRs and mannose receptors, that make them efficient at capturing and responding to antigens. They also express high concentrations of MHC class II molecules and B7 co-stimulatory molecules. By expressing chemokine receptors similar to T lymphocytes, they have the ability to localize in T lymphocyte regions of lymphoid tissue. By colocalizing to these areas, they are uniquely positioned to present antigens to recirculating T lymphocytes. Antigen-presenting dendritic cells function to capture antigen and then migrate to T lymphocyte areas of secondary lymphoid organs, where they present fragments of the antigen on their surface and increase their expression of co-stimulatory molecules that activate T lymphocytes. Specifically, migrating dendritic cells, derived from Langerhans’ cells that have captured antigen, enter the lymph node through efferent lymphatics and localize in lymphoid organs, where they present antigenic peptides to T lymphocytes that facilitate B lymphocyte activation and the production of antibody-secreting plasma cells. In addition to their function as antigen-presenting cells, they are also important in the process of negative selection in the thymus and in the maintenance of peripheral tolerance. The four types of antigen-presenting dendritic cells and their locations are listed in Table 5-3. Circulating dendritic cells, also known as veiled cells, make up less than 1% of peripheral blood mononuclear cells. The second type of dendritic cell, the follicular dendritic cell, is primarily located in lymphoid follicles. These cells are not derived from the bone marrow, do not express MHC class II molecules, and do not function as an antigen-presenting cell. Follicular dendritic cells have FcR and receptors for C3b. They store antigen-antibody and antigen-C3b complexes and are thought to be involved in the development and maintenance of memory B lymphocytes. TABLE 5-3 Antigen-Presenting Dendritic Cells and Their Primary Location Fig. 5-10 Dendritic cell functions. NK cells are nonspecific cytotoxic cells that are important in early responses to tumor cells and viral infections. NK cells are bone marrow–derived, large granular lymphocytes that make up 5% to 15% of the peripheral blood mononuclear cells. Their size, slightly larger than that of a small lymphocyte, and the presence of abundant granular cytoplasm distinguish them from T lymphocytes (Fig. 5-11). They are commonly referred to as large granular lymphocytes. The cytoplasm of NK cells and CTL (Tc) is characterized by cytotoxic granules that contain perforin and granzymes, two potent pathways mediating lysis of the target cell. NK cells and T lymphocytes express numerous similar surface molecules and kill virus infected cells and tumor cells by similar mechanisms. Two membrane molecules, CD16 and CD56, are commonly used to identify NK cells. NK cells express FcγR (CD16) and the β-subunit of the IL-2 receptor (CD2). They do not express antigen-specific TCR or CD3 molecules. In contrast to cytotoxic lymphocytes, NK cells are not MHC restricted, are constitutively cytolytic, and do not develop memory cells. Because NK cells are activated early in an immune response and do not require a previous sensitization phase to develop memory cells after activation, they are the cytotoxic cell of innate immunity, the counterpart to the adaptive immune response’s CTL (Tc). Although NK cells do not express any antigen-specific molecules, they are very efficient at recognizing and killing altered or virally infected cells. NK cell activity is regulated through activating and inhibitory receptor molecules expressed on their cell surface (Fig. 5-12). These NK cell receptor molecules fall into two distinct categories: the immunoglobulin-like NK receptors and the C-type lectin-like NK receptors. Ligands for these receptors are cell surface molecules whose expression has been altered as a result of infection or damage. Ligands for activating receptors that stimulate NK cell activity commonly include viral and stress-induced proteins. Ligands for inhibitory receptors that block NK cell activity most commonly involve class I MHC molecules. A decreased expression of class I MHC molecules makes cells susceptible to NK cell–mediated lysis. A decrease in MHC class I expression often occurs in virus-infected cells and in neoplastic cells, making them susceptible to attack by NK cells. Normal cells are protected from NK cell killing because all nucleated cells express class I MHC molecules. This is an oversimplification of the “opposing-signals” model of NK cell regulation of how cytotoxic activity is limited to altered self-cells. Recent studies on the molecular mechanisms of NK cell regulation indicate that the absence of an inhibitory stimulus by itself is insufficient for triggering NK cell killing. NK cells also require triggering of activating receptors. Several activating receptors have been identified. One is the NKG2D receptor, a C-type lectin-like molecule that recognizes a number of stress-induced proteins. These stress-induced proteins are normally only constitutively expressed in the intestinal epithelium or as a result of cellular distress caused by infection or neoplastic transformation. There are a number of additional activating receptors, some of which recognized viral proteins, that are structurally similar to class I MHC molecules. Fig. 5-12 Regulation of natural killer (NK) cell activity through activating and inhibitory receptors. General Properties of Cytokines Cytokines make up a vast group of low molecular weight soluble glycoprotein proteins that are produced by immune and nonimmune cells, are largely produced locally, and act locally to direct the immune response. The expression of cytokine receptors and their respective ligands is highly regulated and contributes to the complexity of the systemic organization of the immune response. Cytokines are involved in every aspect of leukocyte biology and the immune response and are essential to leukocyte development, recirculation, differentiation, and activation and in maintaining self-tolerance. Cytokines can influence the cytokine-producing cell itself (autocrine), other cells present locally (paracrine), or distant cells (endocrine) (see Fig. 12-1). Cytokines use common signal transducing pathways, converting an extracellular signal through a cell surface receptor to activate or inhibit the target cell. The breadth and depth of knowledge regarding the function, regulation, and control of cytokines is overwhelming; however, an overview of the major cytokines and their primary functions is important for understanding the pathogenic mechanism of many disease processes. Some of the major cytokines and their primary biologic activities are presented here and in Chapter 3 as they pertain to acute and chronic inflammatory responses. Cytokines that broadly influence innate and adaptive immune responses include IL-1, interferons (type 1), IL-6, and TNF-α. These cytokines are produced and influence a wide array of cell types. Cytokines that are involved in hematopoiesis and lymphocyte development include IL-2, IL-3, IL-4, IL-5, IL-12, IL-15, TGF-β, and GM-CSF to mention a few. Chemokines are a large group of cytokines that influence leukocyte development, trafficking, and function. They are organized into subfamilies, with distinct functions, based on the position of cysteine residues. The C-X-C subfamily of chemokines is primarily produced by activated macrophages and tissue cells (e.g., endothelium), and the C-C subfamily is largely produced by activated T lymphocytes. Chemokines are responsible for the anatomic localization (“homing”) of lymphocytes within lymphoid and nonlymphoid tissue. Chemokines and the other proinflammatory cytokines are more thoroughly covered in Chapter 3. The most important functional group of cytokines related to the pathogenesis of a number of diseases of immunity are those involved in the regulation of TH lymphocytes. As discussed previously, TH lymphocytes are classified based on their functional capacity and ability to elicit primarily an antibody response or a cell-mediated immune response rather than on their expression of specific cell markers (Fig. 5-13). TH1 lymphocytes are activated by IL-12 and IL-18 and produce primarily IL-2, IFN-γ, and TNF-β to direct a cell-mediated immune response. TH2 lymphocytes are activated by IL-4 and produce primarily IL-3, IL-4, IL-5, IL-6, IL-10, and IL-13 to direct a humoral immune response. As discussed later in the chapter, the type of response (TH1 versus TH2 type) may determine if a diseased state will occur. IL-15 regulates the growth and activity of NK cells. As indicated in Fig. 5-13, some cytokines, such as IL-10 and TGF-β, downregulate immune responses. In summary, cytokines produced by the TH1 or TH2 subset of lymphocytes not only promote the activation and functional capacities of the subset that produces them (autocrine effect) but also inhibit the development and activity of the other subset. This is known as cross-regulation and has important implications with regard to protective immune responses and adverse immune responses, as is discussed later. Fig. 5-13 Cross-regulation of immunity. MHC class I molecules are present on all nucleated cells (and platelets in some species). Their major function is the presentation of peptide fragments of antigens to CTL (Tc) (CD8+). This requirement results in CD8+ lymphocytes being MHC class I restricted. MHC class I molecules are further subdivided into highly polymorphic loci, referred to as Ia, and relatively nonpolymorphic loci, referred to as Ib, Ic, and Id. Each class I molecule is composed of a heterodimer consisting of a polymorphic α-chain that is linked to a nonpolymorphic β2-microglobulin. The extracellular region of the α-chain consists of three domains (α1, α2, and α3). The α1 and α2 domains form a groove where the peptide fragments bind the MHC molecule (Web Fig. 5-1). Although MHC class I molecules differ in their ability to bind peptide fragments, they are not as restrictive as antibodies and TCRs. The intracellular processing of antigen into peptide fragments, the association of those fragments with MHC class I molecules, and their transport to the cell surface is a complex process. Antigen uptake by antigen-presenting cells is by phagocytosis or endocytosis. Antigen processing is the degradation of an antigen into peptide fragments, which are complexed with MHC molecules. Antigen presentation is the transport of the peptide-MHC complex to the membrane, where they are displayed. Antigens can arise intracellularly (endogenous) and extracellularly (exogenous), and the immune system most effectively eliminates these antigens through the elaboration of CTL (Tc) or secretion of antibody. The immune system uses two different processing pathways for antigen processing and antigen presentation. Intracellular antigens are processed in a cytosolic pathway and presented in association with MHC class I molecules (Fig. 5-14). Extracellular antigens are processed in an endocytic pathway and presented in association with MHC class II molecules. Endogenous antigens are degraded into small peptide fragments within the cytoplasm by the proteasome complex. Peptide fragments are transported to the endoplasmic reticulum (ER) by an adenosine triphosphate–binding peptide transporter (TAP). Within the ER, the newly synthesized MHC class I α-chain and associated β2-microglobulin bind the antigenic peptide and form a complex that is transported from the ER to the Golgi and then to the plasma membrane for presentation to CD8+ CTL (Tc). The antigen recognition molecule of the cytotoxic T lymphocyte (TC) recognizes the MHC-peptide complex by means of its CD8 molecule, which functions as a co-receptor, binding to the nonpolymorphic α3 domain of the MHC class I heavy chain. Because CTL (Tc) only recognize peptides when they are presented as a complex with MHC class I molecules, they are referred to as being MHC class I restricted. MHC restriction is the result of positive selection of lymphocytes during T lymphocyte development in the thymus. Portions of some antigens, those portions not processed for presentation, and in some cases, entire antigens are completely degraded by exopeptidases of the antigen-presenting cell into amino acids and do not initiate an immune response. Endogenous antigens are most frequently encountered during viral infections and thus CTL (Tc) are an important defense mechanism for eliminating virally infected cells. Fig. 5-14 Antigen processing and presentation by an antigen-presenting cell, and antigen recognition by T lymphocytes. The MHC influences transplant acceptance or rejection, immune responsiveness, and the pathogenesis of a number of diseases. The MHC represents a complex of genes that encode specialized molecules involved in antigen presentation and thus regulate immune responses. The ability of the immune system to respond to an antigen is determined in part by the binding of peptide fragments to MHC molecules, which are then presented on the surface of antigen-presenting cells. There is a growing body of information associating certain MHC alleles with increased or decreased susceptibility to certain diseases (Web Table 5-2). Because the MHC genes of most domestic species are not well characterized, it is difficult to distinguish if the observed effects are due to the MHC itself or to other tightly linked genes. These conclusions are generally the result of an observation that some MHC alleles occur at a higher frequency among individuals affected with the disease, as compared with the general population. The association of an increased risk with certain MHC alleles is never the sole basis for determining if an individual will develop the disease, as often other hereditary and environmental factors also play an important role. The diseases most often associated with certain MHC alleles have a pathogenesis that incorporates a significant immunologic component. The types of diseases identified are diverse; however, they frequently include autoimmune, infectious, and allergic diseases. Additionally, MHC diversity may increase or decrease the susceptibility to infectious diseases. There is evidence that MHC polymorphisms may significantly impact disease resistance and is best illustrated in species in which there is a loss of MHC diversity attributed to a limited breeding pool of animals. In the case of the cheetah and Florida panther, the current breeding stocks of both species are derived from a limited genetic pool, thus resulting in reduced MHC diversity. In both species, there is an increased susceptibility to infectious agents that is not seen in other species of big cats. WEB TABLE 5-2 Hypersensitivity reactions have historically been classified on the basis of the immunologic mechanism that mediates the disease as type I, type II, type III, or type IV. Types I, II, and III are mediated by antibody, and type IV is mediated by macrophages and T lymphocytes. Type I is also known as immediate type hypersensitivity and most often is the result of an IgE response that is directed against an environmental or exogenous antigen (also known as an allergen). The result is the release of vasoactive mediators from IgE-sensitized mast cells, and these mediators produce an acute inflammatory response. Type II is also known as cytotoxic hypersensitivity and most often occurs when IgG or IgM is directed against either an altered self-protein or a foreign antigen bound to a tissue or cell. The result can lead to either destruction of the tissue or cell by ADCC, complement-mediated lysis, or altered cellular function without evidence of tissue or cell damage. Type III is also known as immune complex hypersensitivity and is due to the formation of insoluble antibody-antigen complexes (also known as immune complexes). The result is activation of the complement system and the development of an inflammatory reaction at the sites of immune complex deposition. Type IV is also known as delayed-type hypersensitivity (DTH) and is the result of activation of sensitized T lymphocytes to a specific antigen. The resulting immune response is either mediated by direct cytotoxicity or by the release of cytokines that act primarily through macrophages. This original classification, as proposed by Gell and Coombs, was based largely on the primary initiating event involved in the individual reactions and not on the actual pathogenesis as it relates to what is seen clinically or pathologically. Although the original classification of hypersensitivity reactions is still valid, “newer” versions that are based on the pathogenesis better illustrate the complexity of these reactions and the specific pathology (lesions) associated with them. For the purposes of our discussion, we will use the original version of the Gell and Coombs classification presented in Table 5-4, understanding that many of the diseases associated with hypersensitivity reactions are actually complex and may involve more than one type. In humans, genetic mapping studies of most diseases characterized by a hypersensitivity reaction suggest that there are disease-associated susceptibility genes, further supporting the complex pathogenesis of these diseases. Finally, the pathogenesis of many diseases rarely involves a single hypersensitivity reaction, and in fact, some diseases may begin as an immediate hypersensitivity but progress to be predominantly DTH. For clarity, the hypersensitivity diseases are discussed in the context of their predominant mechanism except when it is appropriate to elaborate on the progression of a disease.
Diseases of Immunity
Innate Immunity (Nonspecific Immunity)
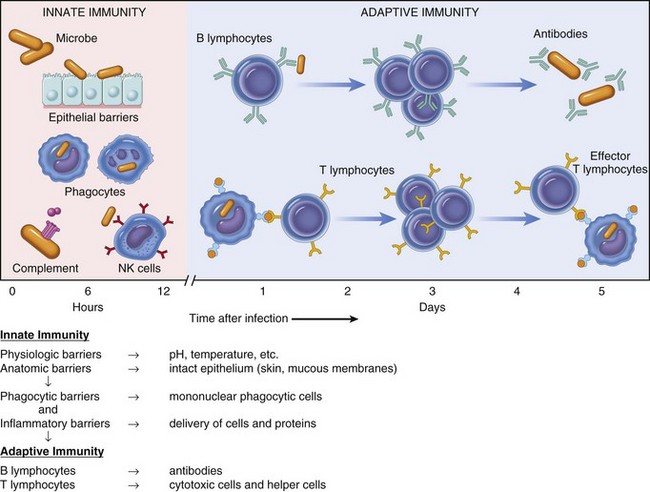
NK, Natural killer. (From Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
Recognition Molecules of Innate Immunity
Toll-Like Receptors
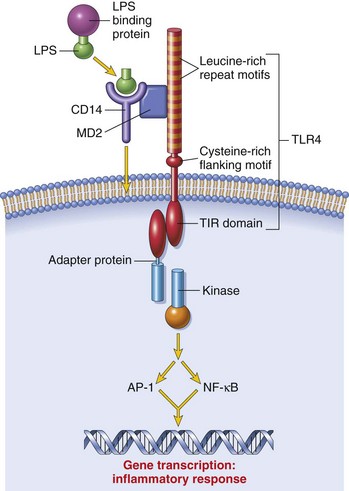
The binding of LPS to TLR4 results in activation of a signal transduction pathway, leading to gene transcription and the elicitation of an inflammatory response. AP-1, Activating protein 1; NF-κB, nuclear factor-kappa B; TIR, Toll/interleukin-1 receptor. (From Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
Adaptive Immunity (Specific Immunity)
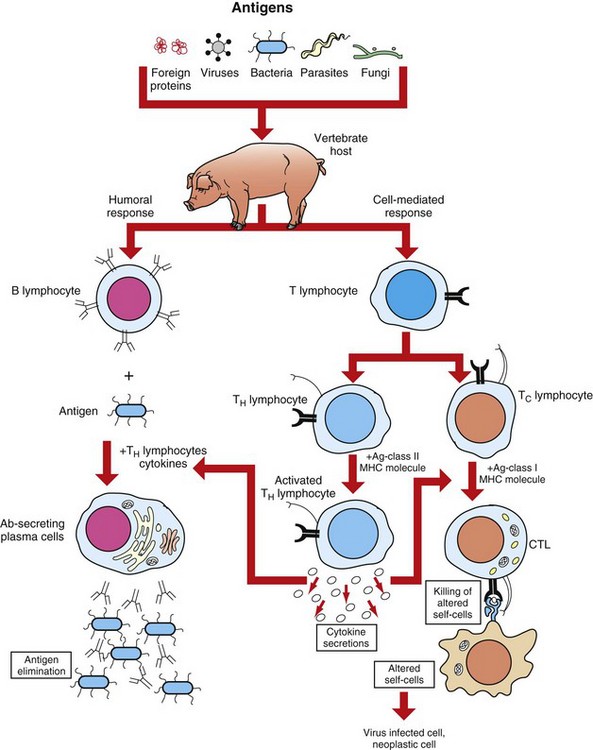
CTL, Cytotoxic T lymphocyte. (Adapted from Goldsby RA, Kindt TJ, Osborne BA: Kuby immunology, ed 4, New York, 2000, WH Freeman.)
Cells and Tissues of the Immune System
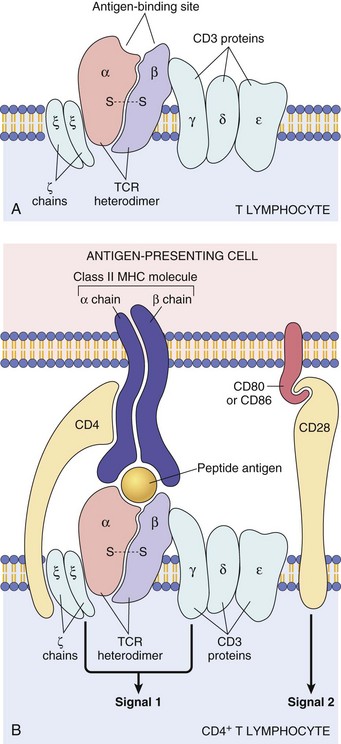
A, TCR-α and TCR-β chains complexed with CD3 γ-, δ-, and ε-chains and the invariant, ζ-chains. B, Illustrating how the TCR recognizes antigen in the context of major histocompatibility complex on the antigen-presenting cell (top) and how the ζ-chains and CD3 γ-, δ-, and ζ-chains deliver one of the two required signals for activation of the T lymphocyte. The second required signal is delivered by the co-stimulator molecules CD28 on the T lymphocyte and B7 on the antigen-presenting cell. MHC, Major histocompatibility complex. (A from Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders. B from Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
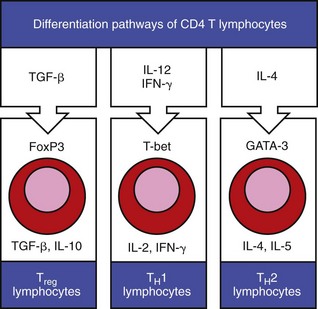
Cytokines in the extracellular tissue fluids direct the differentiation of CD4 T lymphocytes to specific secretory types, such that they secrete specific cytokines that have specific functions on other lymphocytes in the environment. (Adapted from Parham P: The immune system, ed 3, 2009, Garland Science.)
Cytokine
TH1
TH2
IL-2
+
IFN-γ
++
TNF-β
++
GM-CSF
++
+
IL-3
++
++
IL-4
++
IL-5
++
IL-13
++
Function
Antibody*
+
++
IgE
++
Eosinophils, mast cells
++
Macrophages
++
Type IV hypersensitivity
++
Cytotoxic T lymphocytes
++
B Lymphocytes
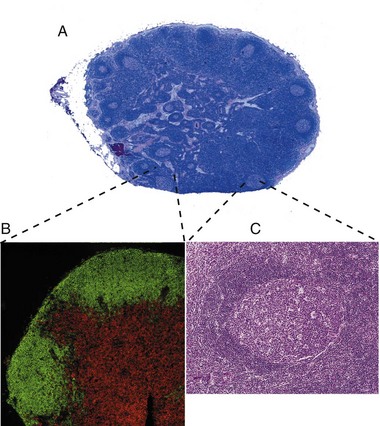
A, Outer cortex, containing numerous secondary lymphoid follicles with characteristic pale centers, and inner medulla are easily identifiable. B, Localization of B lymphocytes (labeled with a B lymphocyte marker conjugated with a green fluorochrome) and T lymphocytes (labeled with a T lymphocyte marker conjugated with a red fluorochrome). Immunofluorescence photomicrograph. C, Higher magnification of a secondary lymphoid follicle illustrating the central pale, germinal center, which contains primarily proliferating B lymphocytes, CD4+ lymphocytes and dendritic cells, surrounded by densely packed small B lymphocytes. H&E stain. (A, B, and C from Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
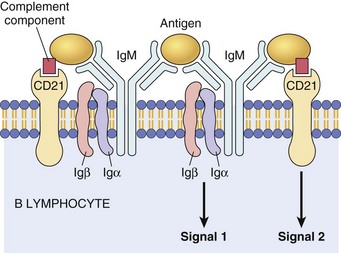
Membrane IgM (or IgD, not shown) and the signaling molecules Igα and Igβ. CD21, also known as complement receptor-2, binds complement components and activates B lymphocytes. Ig, Immunoglobulin. (Courtesy Dr. Alex McPherson, University of California, Irvine.)
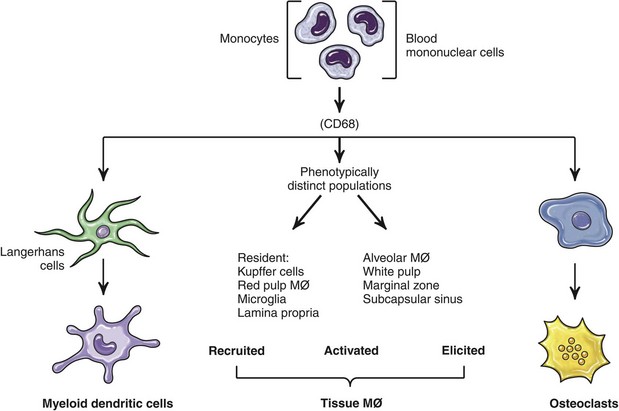
Mononuclear Phagocytic System (Monocyte-Macrophage System)
Organ/Tissue
Name
Location
Lung
Alveolar macrophages
Pulmonary intravascular macrophages
Alveolar spaces
Capillaries of the lung
Connective tissues
Histiocytes
Interstitium
Kidney
Mesangial cells
Glomerular tuft
Brain
Microglial cells
Neuroparenchyma and perivascular areas
Bone
Osteoclasts
Bone marrow
Blood
Monocytes
Circulation
Liver
Kupffer cells
Hepatic sinusoids
Macrophages
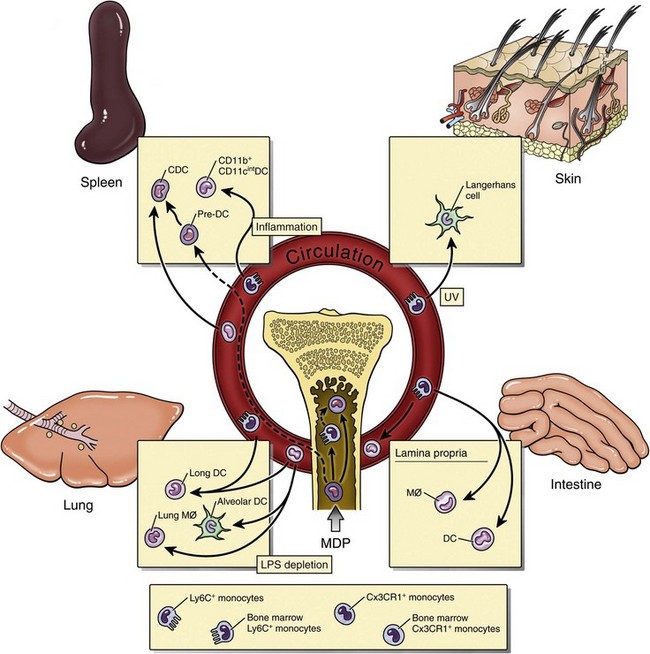
Dendritic Cells
Dendritic Cells
Location
Langerhans’ cells
Skin, mucous membranes, iris, ciliary body
Interstitial dendritic cells
Most major organs
Interdigitating dendritic cells
T lymphocyte area of secondary lymphoid tissue and thymic medulla
Circulating dendritic cells
Peripheral blood
Specialized dendritic cells in the epidermis (Langerhans’ dendritic cells) capture antigen via phagocytosis or endocytosis and migrate to regional lymph nodes, where they present peptide fragments of the antigen to naïve T lymphocytes. (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Natural Killer Cells
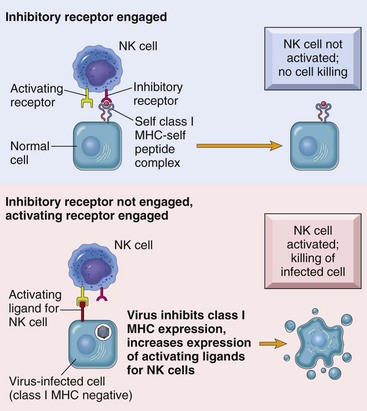
MHC, Major histocompatibility complex. (From Kumar V, Abbas AK, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
Cytokines: Messenger Molecules of the Immune System
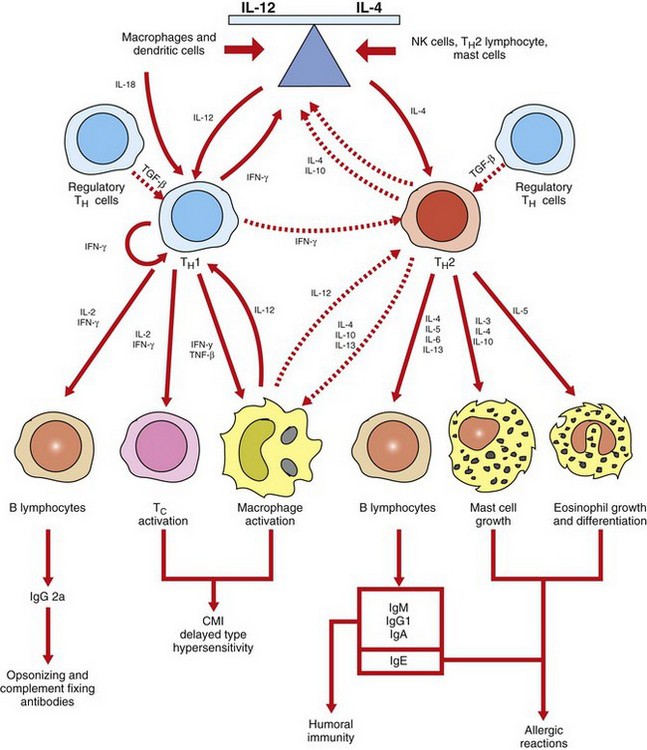
Cross-regulation of TH1 and TH2 lymphocytes in part determines if an immunity is primarily a cell-mediated response or a humoral response. TH1 lymphocytes, activated primarily by IL-12 and IL-18, promote cell-mediated immunity (CMI) by activating macrophages and cytotoxic T lymphocytes. TH2 lymphocytes, activated primarily by IL-4, promote humoral immunity by producing cytokines that activate B lymphocytes to develop into antibody-secreting plasma cells. TH2 lymphocytes also produce cytokines that activate mast cells and eosinophils in the pathogenesis of allergic diseases. The cross-regulation of TH1 and TH2 lymphocytes provides an inverse relationship between cell-mediated and humoral immunity. IFN-γ, Interferon-γ; Ig, immunoglobulin; IL, interleukin; NK, natural killer; TNF-β, tumor necrosis factor-β. (Adapted from Goldsby RA, Kindt TJ, Osborne BA: Kuby immunology, ed 4, New York, 2000, WH Freeman.)
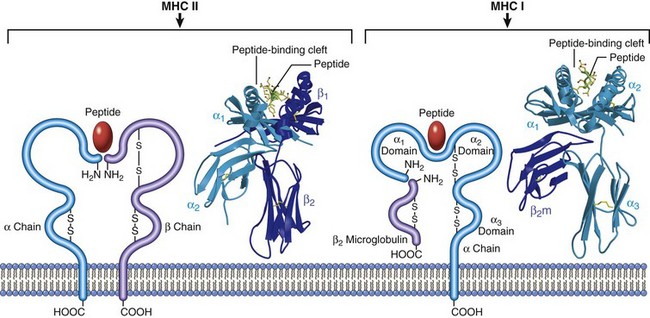
Structure and Function of Histocompatibility Antigens
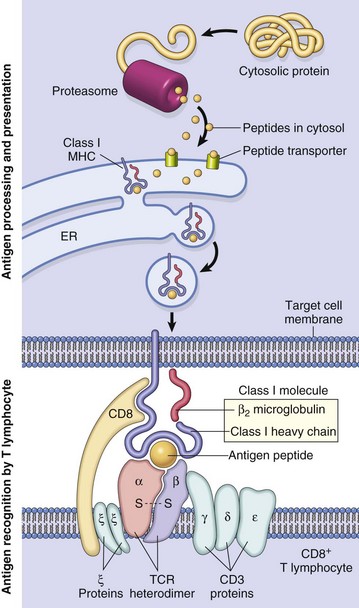
A class I major histocompatibility complex (MHC)–restricted CD8+ lymphocyte is depicted. ER, Endoplasmic reticulum; TCR, T lymphocyte receptor. (From Kumar V, Abbas AK, Fausto N: Robbins & Cotran pathologic basis of disease, ed 7, Philadelphia, 2005, Saunders.)
Major Histocompatibility Complex and Disease Association
Disorders of the Immune System
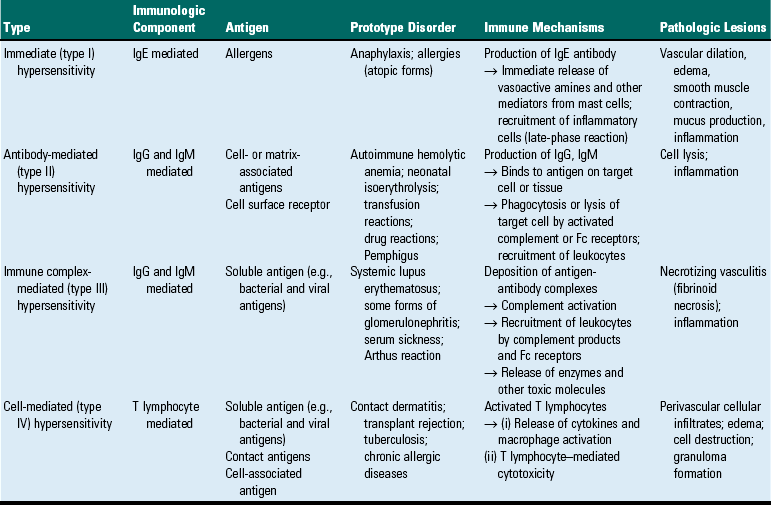
Mechanisms of Immunologic Tissue Injury: Hypersensitivity Reactions
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diseases of Immunity