Urogenital Surgery in Camelids
Urinary Obstruction
Urolithiasis in camelids is not as well recognized as in cattle and small ruminants. Information about uroliths in camelids often is extrapolated from experience with other ruminants, including cattle, sheep, goats, and camels. In general, information from other species is helpful in managing camelids with urolithiasis, but a few important anatomic differences exist. The predisposition to obstructive urolithiasis in male camelids involves a combination of anatomic and dietary factors. Uroliths in male camelids most frequently lodge at the reflection of the urethra around the ischium or at the point where the penile urethra narrows to enter the glans penis.1 Urethral obstruction at these sites may result in rupture of the urethra or the urinary bladder. Camelids do not commonly have obstructions in the sigmoid flexure, as do cattle, and they do not have a urethral process (vermiform appendage), as do small ruminants (Figure 60-1).
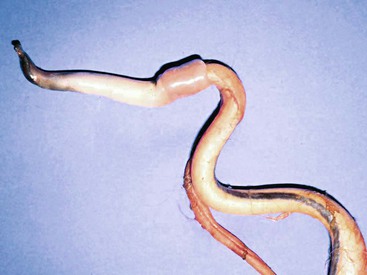
Figure 60-1 Penis of an adult alpaca. Note sigmoid flexure, the attachment of the retractor penis muscles, and the cartilaginous tip projecting from the glans penis.
Struvite (magnesium ammonium phosphate), apatite (calcium phosphate), calcium carbonate, urate, calcium oxalate, and silicate uroliths have all been seen in camelids (Figure 60-2). Struvite and apatite uroliths may be seen in animals fed high-grain diets, whereas animals consuming legumes are predisposed to calcium carbonate uroliths. Silicate stones may be observed in animals grazing silicaceous plants and soils. Calcium oxalate stones may be associated with oxalate containing plants. A significant factor in availability of urolith components and their binding ability is urine pH.1–3 Struvite, apatite, and calcium carbonate uroliths are known to precipitate in alkaline urine.3–6 Struvite crystallization occurs only at a pH range of 7.2 to 8.4, whereas apatite stones develop at a urine pH of 6.5 to 7.5.7 Urine pH may have little or no effect on silicate or calcium oxalate uroliths. Reduced consumption of water or dehydration may contribute to urolith formation by contributing to supersaturation of urine, precipitation of crystals, and formation of organic and inorganic crystalloids in urine.
Medical Therapy
The penis should be examined and exteriorized, if possible. Penile extension is facilitated by use of sedatives. Acepromazine (0.05–0.1 mg/kg, intravenously [IV] or intramuscularly [IM]) and diazepam (0.1mg/kg, slow IV) provide tranquilization or sedation and muscle relaxation.8,9 The use of xylazine should be avoided, when possible, because it promotes diuresis through the inhibition of antidiuretic hormone (ADH) and creation of transient hyperglycemia. Increased urine output may contribute to rupture of the bladder or urethra if the obstruction is not expeditiously relieved. Lumbosacral epidural using 2% lidocaine (1 milliliter [mL]/7 kilogram [kg]) may be utilized in place of sedation in camelids to relieve discomfort and aid in exteriorization of the penis. Once the penis is exteriorized, the distal penis should be carefully examined for the presence of a urolith or a proteinaceous urethral plug. Camelids do not have a urethral (vermiform) process. Rather, the urethral opening is found at the tip of the glans penis adjacent to the fibrocartilaginous projection. The narrowing of the urethra as it enters the glans penis is a common site of obstruction.1 The urethral diverticulum at the level of the ischial arch prevents retrograde passage of a urinary catheter into the urinary bladder.10–12 Therefore, retrograde catheterization or retropulsion of uroliths usually is not successful and is not recommended to avoid risk of further trauma or rupture of the urethra. Attempts at retropulsion of uroliths may result in overdistension of the urinary bladder as the stone is diverted into the diverticulum, allowing fluid to pass into the bladder, followed by the urolith flowing back into the urethra and preventing the bladder from emptying.
Surgical Treatment
Tube cystostomy for urinary diversion in ruminants was initially described in 1965 as a treatment for ruptured bladders in steers.13 Since then, it has become popular as a treatment for obstructive urolithiasis in many species of animals, especially in small ruminants. In small ruminants treated with tube cystostomy, favorable long-term success rates have been reported.14,15
Tube cystostomy provides an alternative surgical treatment to the perineal urethrotomy and urethrostomy techniques previously used to manage urinary obstructions. Long-term outcome of urethrostomy and urethrotomy are reported to be poor in small ruminants, and clinical experience suggests similar complication rates, as observed in goats.16,17 Suboptimal outcome is attributed to stricture formation at the urethrotomy or urethrostomy site. The resulting urethral obstruction may be debilitating and leave limited options for revision. Also, urinary diversion techniques such as prepubic urethrostomy and perineal urethrostomy are unsuitable for breeding animals because they cause loss of penile function and patency.16,18 Urinary bladder marsupialization has been described in pygmy goats but has been associated with extensive urine scalding, stoma stricture, and bladder prolapse through the fistula site.19,20 Laser lithotripsy, although sometimes used in equine urolithiasis, has been described in only a few case reports in ruminants.21–24
To date, the most successful surgical method of treating obstructive urolithiasis in ruminants and camelids is surgical tube cystostomy.13,14,25 In this technique, the patient is anesthetized in dorsal recumbency, and the bladder approached via a paramedian incision. A cystotomy is performed to remove any uroliths present, and the urethra is flushed in a normograde fashion with saline, if possible, and retrograde flushing may be attempted at that time. Then, a Foley catheter (12 to 24 French [Fr]) is passed through a stab incision in the body wall of the inguinal region. The catheter tip is inserted into the bladder through a stab incision, the cuff inflated, and a purse-string suture placed in the wall of the bladder (Figure 60-3). The cystotomy incision is closed in inverting continuous suture patterns (Cushing or Lembert). Following closure of the celiotomy incision, the external portion of the Foley catheter is secured to the skin of the ventral abdomen with sutures or bandages. The catheter is allowed to drain freely for 7 to 10 days, after which time it is periodically occluded to test for normograde urine flow through the urethra. Periods of occlusion are limited so as to minimize the risk of further trauma to the urethra. Once normograde flow is established, the catheter balloon is deflated, and the catheter is removed. The goal of this procedure is to allow for urine diversion from the bladder so that the urethra is rested. The rest period allows for relaxation of urethral spasms, decrease in inflammation, resolution of edema, and dissolution of the uroliths through urine acidification. The obstructing urolith can then be passed by the animal. In small ruminants, urine flow is reestablished a mean of 11 days after surgery.14,25 Success of this procedure has been reported to range from 76% to 90% in the short term, and 86% in the long term.14,25 Success rates have been shown to be dependent on several factors.16,25 The major drawback of surgical tube cystostomy is the cost associated with the procedure. Tube cystostomy has been used to restore breeding function in intact males, avoiding the loss of breeding function as previously described.26 Clinical experience with this technique has been successful in llamas, alpacas, and a camel. To reduce the costs associated with the tube cystostomy procedure, a percutaneous tube cystostomy technique has been developed in small ruminants for placement of the cystostomy tube.27 This technique was successful in the initial description, but subsequent reports indicated that it is 5.6 times more likely to require a second procedure to replace the tube following premature tube loss.15 Vesicular irrigation with hemiacidrin has been used in conjunction with percutaneous tube cystostomy to restore urinary function in a goat.27 The authors concluded that irrigation resulted in rapid dissolution of calcium phosphate uroliths without the need for celiotomy and cystotomy. Vesicular irrigation could be combined with surgical or percutaneous tube cystostomy and perhaps also with other techniques to dissolve uroliths in situ. The efficacy of this technique on different types of uroliths is not known.
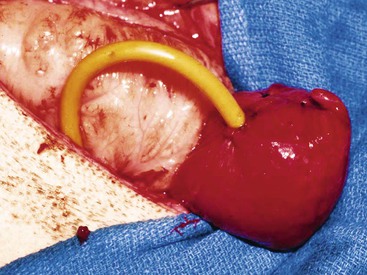
Figure 60-3 Tube cystostomy procedure. Note that the cystotomy incision is sutured closed and a purse strong suture is in place surrounding the entrance of the Foley catheter into the bladder.
Urinary bladder marsupialization has been moderately successful in restoring urination in goats.19,20 This procedure was used successfully in a llama suffering a ruptured urethra at the level of the ischial arch. Prepubic cystostomy eliminates urinary continence and may, as a result, be unacceptable to some owners. In this technique, the apex of the bladder is marsupialized to the ventral body wall along the ventral midline via a midline celiotomy. A stoma is created, and it allows for continuous drainage of urine from the bladder. Complications of this procedure may include bladder mucosal prolapse through the stoma, urine scald on the ventrum of the animal, stricture of the stoma, and ascending urinary tract infections through the marsupialization site.19,20 Published success rates for small ruminants range from 66% to 94%.19,20 Hospitalization is expected to be shorter than tube cystostomy (mean of 4 days versus 14 days), which may reduce cost associated with the procedure.
Laser lithotripsy for disruption of uroliths in large animal species has been reported using a chromium–thulium–holmium:yttrium–aluminum–garnet (Ho:YAG) laser.23,24 The use of lithotripsy is dependent on the presence of a urethra sufficiently large for passage of the laser via an endoscope, and positioning of the uroliths in the distal urethra. Type of urolith may also affect success rates. Lithotripsy may present a viable option for removal of uroliths in camelids, but clinical use of this technology has not been reported in llamas or alpacas.
Mucosal grafting may be viable for the repair of ruptured urethra.28 In this technique, the urethra is reconstructed using a strip of tissue, for example, from the buccal. Buccal mucosa has been easily accessible tissue for this procedure.
Perineal urethrostomy has long been the mainstay of urolithiasis treatment in ruminants but it is considered a salvage technique in most cases, although some animals have survived for long periods after surgery. With this technique, a permanent opening is made in the urethra proximal to the site of obstruction through which the animal excretes urine. Perineal urethrostomy does not guarantee urethral patency, as an animal may still experience an obstruction proximal to the stoma. As a result, the urethrostomy stoma should be placed as proximal as possible to maximize the diameter of the urethra. Subischial urethrostomy may be the location of choice to optimize urethral lumen, eliminate tension on the penis, and minimize risk of stricture. The most common complication of urethrostomy is stricture of the stoma, which may occur within 6 months following surgery.29
Penile amputation (penectomy) may be done during perineal urethrostomy, and this procedure has been described in camelids.1 In this procedure, the penis is transected, and the distal segment may be completely removed.
In early stage cases of urethral obstruction and when the urolith is in a location that can be identified, a simple urethrotomy may be performed over the urolith itself or adjacent to the urolith in healthier tissue. Following incision, the urolith is removed with forceps. An alternative technique involves retropulsion of a urolith from the distal urethra to an ischial urethrotomy, in which it may be removed. Urethral stricture formation is a common side effect of urethrotomy, but the simplicity of the procedure makes it popular in breeding animals where the penis must remain intact.29 Urethrotomy has been described in camelids, with varying success for return to breeding.1 The urethrotomy incision may be allowed to heal without suturing, as suturing may increase the likelihood of stricture formation. Some surgeons, however, recommend suture closure of the urethrotomy as soon as possible to prevent stricture formation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



