Clinical Pathology
Hematology
Erythrocytes
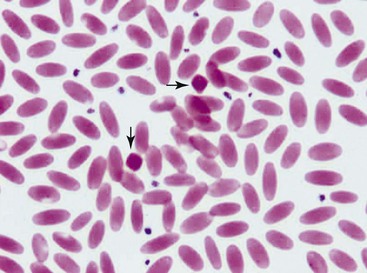
The unique morphologic features of camelid erythrocytes include their elliptical, flat shape and small size. These features are adaptive to life at high altitude as well as arid environments as they provide resistance to osmotic lysis, decreased deformability, and high affinity for oxygen.1–3 Other features of erythrocytes such as hemoglobin crystals and Cabot rings have also been described, but neither an adaptive function nor pathology is known for them. Hemoglobin crystals may appear as darkly eosinophilic, dense, diamond-shaped structures on blood smears from clinically healthy, nonanemic camelids (Figure 31-1). Cabot rings are less common but are also occasionally seen in erythrocytes from clinically healthy camelids. These are threadlike structures in figure of eight or ring-shaped forms within red blood cells (RBCs). They have no known clinical significance and have been described in other species as possibly denatured membrane proteins or a combination of histones and nonhemoglobin iron.4–6
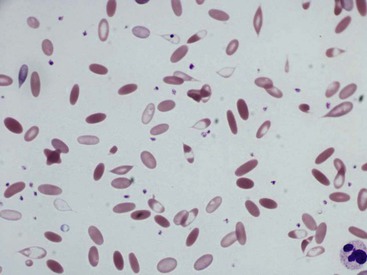
Camelid erythrocyte morphologic abnormalities also include dacryocytes (or tear-drop shaped cells), spindle-shaped erythrocytes, and uneven distribution of hemoglobin in the cells (Figures 31-2 and 31-3). These have been reported as features of iron-deficiency anemia but are also seen in other cases of marked anemia.71
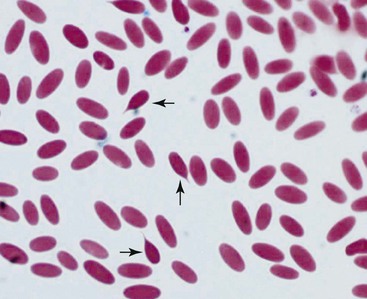
Figure 31-2 Dacryocytes in alpaca blood smear. Tear-drop shaped erythrocytes are seen in normal camelids.
Normal Red Blood Cell Parameters
Packed cell volumes (PCVs) of healthy camelids are usually slightly lower than those of other herbivores, as camelids have higher RBC numbers but a smaller erythrocyte size. The mean corpuscular volumes (MCVs) of camelids is reported to be 21 to 28 femtoliters (fl oz).2
Reference intervals for erythrocyte parameters in camelids appear to be influenced by environmental factors, specifically, altitude, and it would not be appropriate to use some of the published reference intervals when evaluating animals kept at lower elevations.3,9–11
Some evidence indicates that epinephrine release during stressful handling or excitement may lead to a rapid rise and then a fall of PCV, which suggests that camelids have a splenic reserve of RBCs that may be released into the circulation.1 The mean corpuscular hemoglobin concentration (MCHC) of camelids is generally higher than in horses, cows, and sheep. However, the mean corpuscular hemoglobin (MCH), which is an index of the average amount of hemoglobin (Hg) per RBC, is usually lower than reported in species with larger erythrocytes.2
Anemia
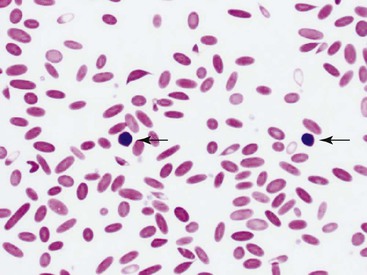
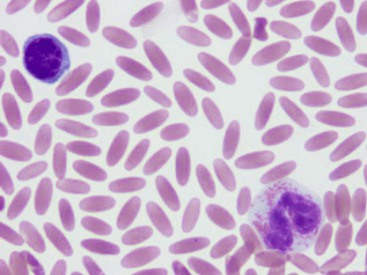
Mild to severe anemia is common in sick llamas and alpacas, often without an underlying cause, even of severe anemia, being identified. A challenge in working up anemia in camelids is the fact that criteria for defining regenerative and nonregenerative anemia are not clear. Anisocytosis, polychromasia, reticulocytosis, and metarubricytosis may all be seen in regenerative anemia, but they are not consistently present. Nonanemic, clinically healthy alpacas and llamas may have reticulocyte numbers in the range of 0% to 1.4%, and 2 to 3 metarubricytes per 100 white blood cells (WBCs) may be seen in nonanemic llamas (Figure 31-4).9,12
An experimental model of anemia in llamas demonstrated that reticulocyte and nucleated RBC numbers increased within 1 week and peaked between 4 and 6 weeks after induction of anemia. Metarubricyte numbers were reported as high as 20 per 100 WBCs during this time.7 Another study of experimentally induced anemia in llamas over a 2-week period resulted in no increase in reticulocytes or metarubricytes.8 In naturally occurring iron-deficiency anemia in llamas, injection of iron dextran resulted in increased PCV and return of the MCV and MCHC to normal, but reticulocyte numbers did not increase above reference intervals.13 In alpacas with experimentally created, acutely severe anemia, reticulocyte percentages increased, starting from an average of 2.6 days after induction of blood loss and reaching a peak of only 1.5% at a mean of 10.4 days after induction of anemia.14 Some of the clinically healthy adult alpacas with optimal diets had not returned to baseline PCV even 3 months after induction of anemia.
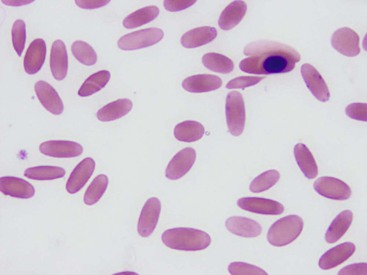
Gastrointestinal and external parasitism may lead to significant anemia caused by blood loss in camelids. Fecal examination, in particular for Haemonchus spp. as well as other gastrointestinal parasites, is always indicated in anemic animals, along with examination of skin for lice and ticks. In addition to blood loss and iron deficiency, specific causes of anemia in camelids include hemoparasite infestations, other causes of hemolysis, and neoplasia. Candidatus Mycoplasma haemolamae (CMhl), a hemotropic mycoplasma, may be identified as small, round or ring-shaped basophilic organism on the margin of erythrocytes. If blood smears are not made promptly after blood collection, the organism typically detaches from the RBC membrane and may appear in the background, where it resembles stain precipitate (Figure 31-5).15 Infection caused by this organism may be associated with mild, moderate, or severe anemia. Use of a highly sensitive polymerase chain reaction (PCR) assay has shown that many, if not most, cases are subclinical. Experimental and natural infections have shown that most healthy, immune-competent camelids that are infected mount an immune response that clears the organism from blood smears and alleviates anemia, if it is present. However, studies have shown that the organism most often is not fully cleared but is either reduced to undetectable numbers or possibly sequestered in other sites. This also occurs in most infected animals treated with oxytetracycline, a commonly used treatment. Camelids that are stressed, immune-compromised, or concurrently infected with other organisms are more likely to become anemic and to show clinical signs of depression, pallor, and lethargy.15 The mode of transmission has not been proven for this infection, although evidence of in utero or periparturient infection is strong.16,17 The prevalence of this organism in camelids in the United States, based on positive PCR amplification, is approximately 30%, with no gender or species predilection.18 Heinz body anemia has been reported in alpacas that had previously ingested red maple (Acer rubrum) leaves. That anemia was characterized by intravascular hemolysis, with numerous Heinz bodies seen on blood smears.19 No other potential causes of hemolytic anemia were identified, which suggests that the red maple leaves caused the Heinz body anemia in these cases. Severe, nonregenerative anemia has been reported with bone marrow neoplasia and myelodysplastic syndromes in camelids.20–22 Cases of anemia in camelids with no other apparent underlying cause may be ascribed to anemia of chronic inflammation. This is probably the most common cause of mild to moderate, nonregenerative anemia overall in domestic animals. The pathogenesis is complex and includes the influence of inflammatory mediators that affect iron utilization, reduce bone marrow responsiveness to erythropoietin, and shorten the survival of erythrocytes.23 Some causes of anemia reported in other species have not been reported in camelids, although they may occur. They include hypophosphatemia and copper toxicity. Llamas and alpacas with signs of copper toxicity and increased liver copper were not found to be anemic.24–26 However, anemia has been seen in llamas with low plasma copper levels. Following copper injections and oral mineral supplementation, plasma copper levels increased, and the PCV returned to within reference intervals.27 Hypothyroidism in some species is commonly associated with mild nonregenerative, anemia, but this has not been proven in camelids. In a report of severe anemia in llamas with low serum thyroxine concentrations, thyroid supplementation did not alleviate the anemia. These llamas were also iron deficient, which is more likely to be the cause of the severe anemia.8 Anemia of chronic renal failure may result from lack of erythropoietin production by the kidneys. This has not been reported in camelids. In a case of a llama with renal failure and a low hematocrit, serum erythropoietin was similar to that in a control llama.
Leukocytes
Adult llamas have a neutrophil-to-lymphocyte ratio of approximately 1.5, which is higher than in ruminants.2,12 A lower neutrophil-to-lymphocyte ratio was reported in a group of 29 alpacas, but these were 10- to 18-month old alpacas, so the difference may be based more on age than on species.28 The ratio may also be affected by an epinephrine-induced or physiologic neutrophilia.
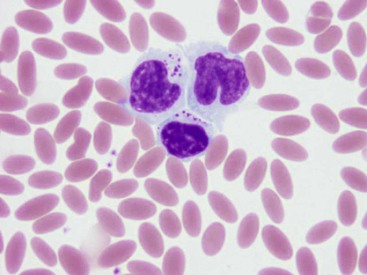
The sizes of camelid lymphocytes are variable, as in ruminants. The percentage of granular lymphocytes in circulation in healthy llamas has been reported to be as high as 30% of lymphocytes. Granules are seen in lymphocytes of varied sizes (Figure 31-6).
Immature neutrophils with band-shaped nuclei may be present with inflammation, as are toxic changes in neutrophil cytoplasm and hyperfibrinogenemia. Less acute or severe inflammation may be associated with neutrophilia with no left shift. Mature neutrophils, a modest increase in band neutrophils, and lymphopenia are seen as part of a stress response, which is common in camelids; stress-induced neutrophilia may take on extreme proportions.9,11
Llama eosinophils are usually hyposegmented with band-shaped to bilobed nuclei. They tend to be present in higher numbers in healthy adult llamas than in other species (Figure 31-7).2,12 Circulating neoplastic leukocytes have been reported infrequently in camelids. In a case of acute myeloid leukemia (AML) in an alpaca, greater than 60% of bone marrow nucleated cells were blasts, and cytopenias and cellular atypia were present in all three hematopoietic cell lines. Cytochemistry and flow cytometry on peripheral blood and bone marrow demonstrated the myeloid nature of the cells, and immunohistochemistry on multiple tissues showed that they contained neoplastic cells.20 In another case of severe anemia and leukopenia in a 2-year-old alpaca, bone marrow evaluation confirmed myelodysplastic syndrome, rather than AML because less than 30% of the bone marrow nucleated cells were blasts.21 Lymphocytosis with atypical circulating lymphocytes is uncommon in lymphoma in camelids but has been reported.22
Platelets
The platelets of llamas and alpacas are round to irregular, with azurophilic granules usually apparent. Platelet reference intervals are generally the same for llamas and alpacas with a range for both species of approximately 90,000 to 860,000/ µL, which is wider than previously reported.2,29
Thrombocytopenia in camelids has been reported in sepsis and in cases of rattlesnake envenomation.30,31 These reports do not clarify whether the thrombocytopenia was caused by disseminated intravascular coagulation. Platelet counts improved in response to supportive treatment in some of the cases.
Hemostasis
Reference intervals for coagulation tests have been established for healthy adult llamas and alpacas with similar ranges seen in the two species. Prothrombin time (PT) is generally between 6–11 seconds and activated partial thromboplastin time (APTT) is approximately 8 to 22 seconds, which is similar to that in dogs and cats but shorter than in cattle, goats, and horses.29,32
Reports of hemostatic disorders in camelids are quite rare, but a single case of factor VIII deficiency in a newborn alpaca has been reported. This appeared to be a primary factor VIII deficiency, typical of hemophilia A.33
What Constitutes the Ideal Panel?
The most informative tests are as follows:
1. The core electrolytes: sodium (Na), chloride (Cl), and potassium (K)
2. Total carbon dioxide or its equivalent and lactate
3. Nitrogen compounds: blood urea nitrogen (BUN) and creatinine
4. Energy substrates: glucose, triglycerides, nonesterified fatty acids (NEFAs), and β-hydroxybutyrate (BOHB)
5. Protein: total protein, albumin; immunoglobulin is useful in some cases, particularly in neonates or camelids with a suspected immunodeficiency
6. Liver enzymes: aspartate transaminase (AST) and γ-gutamyl transferase (GGT)
Electrolytes
Electrolyte abnormalities in camelids, in general, are less severe than in cattle with similar diseases. Sodium and chloride, the major extracellular ions, tend to rise and fall together. Blood sodium concentrations are generally believed to be under tight hormonal control, whereas chloride concentrations often adapt to maintain electrical neutrality, and thus are affected by acid–base imbalances. The main exception to the concurrent directional shifts, or one associated with concurrent shifts of substantially differing magnitudes, occurs with gastrointestinal obstruction: camelids with obstructions trend toward hypochloremia without hyponatremia, or with much less severe hyponatremia.34 Hypochloremia in these cases may be mild or severe, worsening with completeness of the obstruction, proximity to the pylorus, and duration.
Mild, transient hyponatremia is also present during the initial phases of glucose mobilization and hyperosmolar syndrome.35 This reflects the redistribution of water from the intracellular space to the extracellular space because of the osmotic draw of the glucose.
Hypernatremia and hyperchloremia are much more common compared with decreases in these electrolytes. In an informal survey of Oregon State University clinical pathology data, hyponatremia and hypochloremia were found on 5% and 6% of camelid chemistry profiles, respectively, whereas hypernatremia and hyperchloremia were found on 43% and 28%, respectively. Normal camelids have higher blood concentrations of these electrolytes than normal ruminants or horses, potentially reflecting the physiologic differences between the species and related to water or salt conservation in camelids, and it is apparent, on the basis of the Oregon State University data, that the concentrations of these electrolytes have a strong predilection to climb in sick camelids. In this way, camelids are also quite different from other domestic hoofstock.
Because hypernatremia usually develops more rapidly in camelids than does salt poisoning in other species, rapid correction and an overshifting of fluid into the central nervous system are less of a concern. However, camelids with hyperglycemia should be observed frequently during correction, and treatment discontinued and reassessed on first recognition of signs compatible with cerebral edema, including severe obtundation, head or body tremors, a base-wide stance, or ataxia. Treatment of cerebral edema is covered in Chapter 38.
Acid–Base Disturbances
Acid–base disturbance may be assessed in a variety of ways, including blood gas analysis, measurement of the blood concentrations of total carbon dioxide, lactate, or ketone bodies, and calculations of measured cationic–anionic differences. Respiratory acidosis may be accurately assessed only by measurement of the partial pressure of carbon dioxide (pCO2) in arterial blood. Increases reflect hypoventilation and have been seen with diaphragmatic hernia or paralysis, space-occupying lesions in the chest, pneumothorax, and poor ventilatory efforts from weakness or obtundation.36 It is best treated by removing the underlying condition or by mechanical ventilation. Respiratory alkalosis is rarely more than mild and usually is compensatory for metabolic acidosis. As such, it requires no specific treatment.
Metabolic alkalosis, suggested by a high blood pH and increases in blood bicarbonate or total carbon dioxide concentrations, is most common in camelids with gastrointestinal obstructions.34 It is relatively rare, and was seen in only 5% of the Oregon State University camelid blood chemistry profiles. Affected animals usually have concurrent hypochloremia and hypokalemia. Metabolic alkalosis with concurrent hyponatremia and hypochloremia may also be caused by urinary retention, as occurs with a ruptured bladder or ureter. Mild alkalosis in the absence of these other abnormalities is compensatory for respiratory acidosis or may be from unknown causes. Rehydration and correction of the causative lesion are the major treatments for metabolic alkalosis. Normal saline is considered the best basis for intravenous fluid therapy because it lacks a buffer and is, therefore, more acidifying than most other commercial electrolyte preparations.
The most common acid–base abnormality in hospitalized camelid patients is metabolic acidosis, characterized by a low blood pH and a decrease in blood total carbon dioxide or bicarbonate concentrations. It was present on 33% of the Oregon State University camelid chemistry profiles. Metabolic acidosis is mainly the product of bicarbonate loss, production or absorption of lactate, or ketoacidosis. Bicarbonate loss may occur with diarrhea, salivary loss, or rarely renal tubular acidosis. Bicarbonate loss through diarrhea is rarely severe except potentially in young crias with cryptosporidiosis or coronaviral enteritis.37 Salivary loss can be considerable with esophageal obstruction or other impediments to normal swallowing.
Lactic acidosis may occur with forestomach acidosis, intestinal strangulations, or various types of shock and possibly also with acidosis without dehydration.34,38,39 In camelids with severe hyperglycemia, a small amount of blood lactate may be the result of futile cycling, in which excessive intracellular glucose overwhelms the capacity of the Krebs cycle for oxidative glycolysis, and some glucose is catabolized to lactate. This lactate may subsequently be used for hepatic gluconeogenesis, increasing blood glucose and continuing the cycle.
Ketoacidosis is seen predominantly with increases in BOHB, reflecting a situation of fat mobilization triggered by stress or negative energy balance.40 Both lactic acidosis and ketoacidosis are underappreciated as causes of lethargy and obtundation because the availability of direct measurement of lactate or ketone bodies is limited and because indirect measurement such as anion gap calculation is relatively insensitive in camelids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree