CHAPTER 10 Urinary Tract
Fine-needle aspiration (FNA) of the kidneys and bladder to obtain cells for cytologic evaluation is a simple, rapid, safe, and relatively inexpensive procedure. The primary indications for cytologic examination of the urinary tract include unilateral or bilateral renomegaly, discrete bladder masses or bladder wall thickening, and urethral masses. Cytologic evaluation of the urinary tract can frequently distinguish between common causes of organ enlargement (inflammation, cyst, and neoplasia), direct further diagnostics (e.g., culture or biopsy), or prevent needless surgical intervention (e.g., in cases of metastatic neoplasia).
NORMAL ANATOMY AND HISTOLOGY
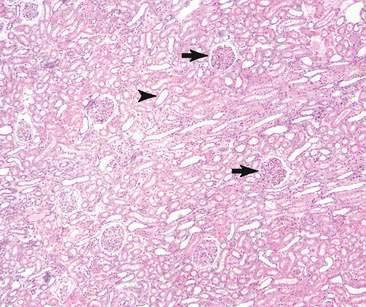
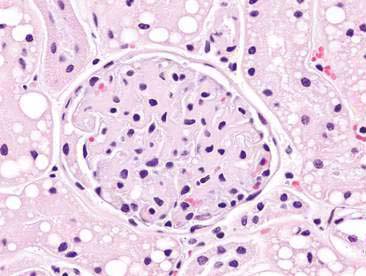
The urinary system is composed of kidneys, ureters, the urinary bladder, and the urethra. The kidney has four basic morphologic components: glomeruli, tubules, interstitium, and blood vessels (Fig. 10-1). The functional unit of the kidney is the nephron. Each nephron is composed of a glomerulus and renal tubule system. The glomerulus is a capillary tuft lined by fenestrated endothelium that is intimately associated with tubule epithelial cells. Components of the glomerulus (Fig. 10-2), including the endothelium, basement membrane, and specialized epithelial cells known as podocytes, make up the filtration barrier of the kidney (Jones et al., 1997).
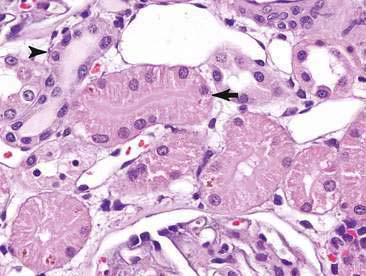
The renal tubule is divided into distinct functional segments including the proximal convoluted tubule, loop of Henle (ascending and descending limbs), distal convoluted tubule, and the collecting ducts. Reflective of function, the epithelial cells lining the tubules vary from a single layer of cuboidal cells with a brush border in the proximal convoluted tubules to columnar epithelium with no brush border in the distal convoluted segments (Fig. 10-3). Caudate transitional cells line the renal pelvis and calyces. Similarly, the mucosa of the ureters, urinary bladder, and urethra are lined almost exclusively by transitional epithelial cells. Renal interstitial tissue is composed of connective tissue (mesenchymal cells), an extensive capillary network, lymphatic tissue, and smooth muscle cells (Jones et al., 1997; Borjesson, 2003).
SPECIALIZED COLLECTION TECHNIQUES
Sampling method for obtaining cytologic specimens depends on lesion location but may involve either direct mass FNA or traumatic catheterization in the case of a urethral mass or bladder mass in the area of the trigone. Conversely, renal biopsy is frequently indicated in dogs and cats with glomerular disease (and proteinuria) or acute renal failure. Biopsy can be performed with ultrasound guidance or using surgical methods. The increased risks associated with biopsy include the transection of blood vessels or the renal pelvis with resultant hemorrhage, hydronephrosis, or hematuria (Borjesson, 2003). A recent multi-institutional study found complication rates for renal biopsy at 13.4% and 18.5% of dogs and cats, respectively (Vaden et al., 2005). The most common complication was hemorrhage. There have been a number of recent published reviews comparing renal biopsy methods to optimize specimen procurement and minimize biopsy-induced complications (Vaden et al., 2005; Rawlings et al., 2003; Vaden, 2004).
For ultrasound-guided aspiration, the patient is maintained in dorsal recumbency. If changes are bilateral, aspiration of the caudal pole of left kidney is recommended as it decreases the risk of accidental aspiration of bowel and pancreas. The needle is directed from the cortex of the caudal pole, ventral to dorsal. The needle can be angled from medial to lateral to avoid hitting a large renal vessel. Care should be taken to avoid hitting the renal pelvis. Begin with 25- to 27-gauge, 1.5-inch needle. If sample of adequate cellularity is not obtained, a larger bore needle can be used. The best results are obtained if multiple preparations are made and one slide is rapidly stained with either a Romanowsky stain or New methylene blue to assess sample adequacy (Borjesson, 2003).
Regardless of the type of mass, aspiration of both the central and peripheral areas is recommended. Frequently the center of a mass may consist solely of necrotic debris or inflammatory cells resulting in a nondiagnostic sample. Although purulent inflammation and necrosis can be associated with malignancy, abundance of either frequently masks the primary disorder. Thus, multiple aspirations in different areas of the mass are almost always recommended to maximize cellular yield and diagnostic potential as well as to differentiate between primary and secondary inflammation (Borjesson, 2003). If fluid is obtained, a direct smear can be made immediately and the remaining fluid can be placed into EDTA to prevent clotting. Both sediment and cytocentrifuged smears can then be prepared, especially if the sample is of low cellularity. Finally, impression smears can be made from renal biopsy specimens. Cytologic evaluation of impression smears can aid in rapid diagnosis of infectious agents or neoplasia (Borjesson, 2003).
Cells from masses within the bladder can be readily obtained using ultrasound-guided FNA or traumatic urethral catheterization. Occasionally, tumor cells can be noted in urine sediment; however, the submission of urine for cytology rarely results in a definitive diagnosis of neoplasia. Traumatic urethral catheterization can provide adequate and diagnostic samples; however, many of the cells obtained may be superficial and reactive transitional epithelial cells. As such, traumatic catheterization can result in a false negative cytology report due to sample bias with the primary mass not being successfully sampled. Although a few cases of tumor implantation along the ventral abdominal wall following direct FNA of bladder masses have been reported (Nyland et al., 2002), this complication is rare and likely does not affect the clinical outcome. As such, ultrasound-guided FNA may remain the best method for obtaining tissue-associated cells and maximizing cellular yield for cytologic review.
NORMAL RENAL CYTOLOGY
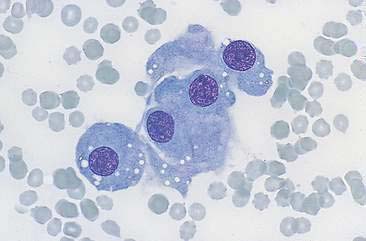
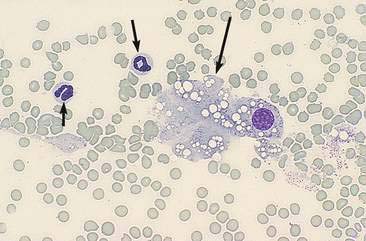
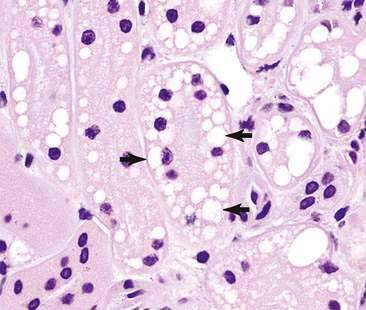
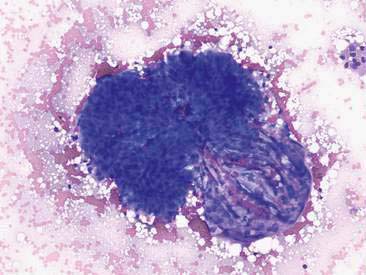
Renal aspirates are typically of low cellularity and usually contain small clusters of renal tubular cells admixed with blood. As the kidneys are highly vascular, blood is generally due to iatrogenic hemorrhage at the time of sampling. Tubular cells generally exfoliate singly or in small clusters of round to oval to columnar epithelial cells. They have abundant basophilic, occasionally vacuolated, cytoplasm that surrounds a uniform, round, often eccentrically placed nucleus (Fig. 10-4). Feline tubular cells are cytologically similar except that cats normally have lipid deposition within their renal tubules. Lipid droplets appear as prominent, variably sized, intracytoplasmic, clear, punctate vacuoles (Figs. 10-5 and 10-6). Fully intact renal tubules arranged in cohesive linear structures may also be present (Fig. 10-7). Tubular cells can also contain dark, intracytoplasmic granules that should not be confused with a well-differentiated melanocyte tumor (Fig. 10-8). Glomeruli may exfoliate singly or in dense, deeply basophilic, rounded clusters, and have very uniform round to oval nuclei (Fig. 10-9).
NON-NEOPLASTIC AND BENIGN LESIONS OF THE URINARY TRACT
Bladder
Polypoid Cystitis, Transitional Cell Polyps, and Papilloma
Hyperplastic and benign mass lesions of the bladder are an uncommon but important subset of bladder diseases that may mimic malignant neoplasia. Polypoid cystitis is characterized by inflammation, epithelial proliferation, and development of a non-neoplastic mass. Similar to most diseases of the urinary bladder, these dogs present with hematuria or recurrent urinary tract infection. However, unlike transitional cell neoplasia, these masses are most frequently located cranioventrally in the bladder rather than in the trigone region (Martinez et al., 2003).
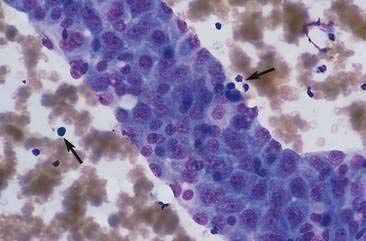
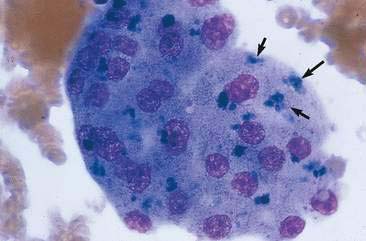
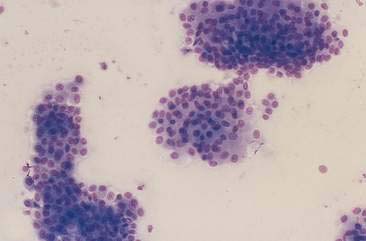
Transitional cell hyperplasia and benign transitional cell polyps can exfoliate in large cellular sheets with mild pleomorphism (Fig. 10-10). Nuclei are generally uniform with coarse, ragged chromatin patterns (Fig. 10-11). Transitional cell papillomas may also occur and are characterized by varying size clusters of uniform transitional epithelial cells. These cells are cuboidal to polyhedral and show mild anisokaryosis with an increased nuclear-to-cytoplasmic ratio (Fig. 10-12). Differentiation between the benign processes of hyperplasia, polyps, and papillomas cannot be made cytologically. Often these processes can be difficult to differentiate from neoplastic processes because close proximity to urine causes mild to marked cellular disruption, making definitive identification problematic (Fig. 10-13).
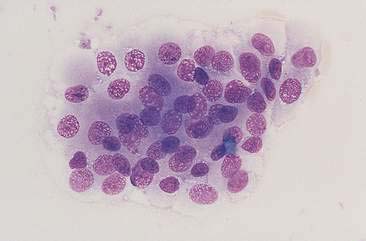
FIGURE 10-11 Transitional cell hyperplasia or benign polyps. The transitional cells within this epithelial cluster have round to oval uniform nuclei. The chromatin is coarse (a common cytomorphologic feature of the urothelial system) without obvious nucleoli. Cell-cell borders are often readily observed and there is only mild pleomorphism. Contrast the features of this cell cluster to the cells in Figures 10-20 to 10-24. (Wright-Giemsa; HP oil.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree