CHAPTER 13 Musculoskeletal System
Lameness is the cardinal clinical sign associated with disease of the musculoskeletal system. Other signs include stiffness, ataxia, weakness, pain, fever, limb and joint swelling, and deformity. Depending on the type of disorder, other organ systems may also be involved, including neurologic, endocrine, urologic, hemolymphatic, digestive, respiratory, and cardiovascular systems. Because of this, an animal with musculoskeletal disease may present with a variety of problems and signs.
SYNOVIAL FLUID EVALUATION
As with other body cavity effusions, a complete fluid analysis is helpful when evaluating a synovial effusion. Routine synovial fluid analysis should include evaluation of color, transparency, protein concentration, viscosity, mucin clot test, nucleated cell count, differential, and cytologic evaluation. These tests are discussed in further detail below. If the sample is limited, the most important component of analysis is the cytology. Typical results for different kinds of joint disease are shown in Table 13-1.
Sample Collection and Handling
Collection of synovial fluid varies to some degree depending on the joint sampled. Descriptions of approaches to various joints have been described. In general, collection of synovial fluid requires the following materials: 3- to 6-mL syringe, 18- to 22-gauge 1-inch needles, and red-top and/or lavender-top tubes. The amount of restraint and necessary levels of sedation and anesthesia will vary from animal to animal. Enough restraint should be used to minimize struggling during collection. In general, many animals will require at least some degree of sedation or anesthesia. Sterile technique is critical when preparing the site and during aspiration. The fur should be clipped and the area of aspiration scrubbed. Care should be taken not to scratch the articular surface during needle insertion. Palpation and slight flexion or hyperextension of the joint will help to identify insertion points of the needle. Aspiration sites for specific joints are described below. The location of aspiration varies with the joint aspirated. The coxofemoral joint can be aspirated cranioproximal to the trochanter major and slightly ventral and caudal. The stifle should be flexed when aspirated. Aspiration can occur medial or lateral to the patellar ligament, midway between the tibia and femur. The tarsocrural joint can be aspirated by hyperextending the joint and inserting the needle lateral or medial to the fibular tarsal bone. To aspirate the shoulder, insert the needle 1 cm distal and slightly caudal to the acromion process. The elbow should be hyperextended and the needle inserted lateral and along side the olecranon. The carpal joints can be simply aspirated by flexing the joint and palpating the joint space. The needle should be advanced slowly through the joint capsule into the joint cavity. The amount of fluid withdrawn depends on the size of animal and joint as well as the amount of effusion present. Synovial fluid will be aspirated easily if there is a significant effusion but a few drops may be obtained from joints without an increase in synovial fluid volume. Before removing the needle from the synovial cavity, the plunger of the syringe should be released to remove any negative pressure. Normal synovial fluid has a gel-like consistency that should not be mistaken for a clot. The gel-like consistency will become less viscous when shaken and return to the original viscosity upon standing; this property is referred to as thixotropy. Clotting is likely to occur if there is significant blood contamination and inflamed joints may form fibrin precipitates or clots. For these reasons, some joint fluid should be put into an EDTA tube (lavender-top tube). The EDTA will interfere with tests such as the mucin clot test and culturing. The synovial fluid should be refrigerated if not immediately evaluated. For samples that may be cultured dependent on the cytologic findings, the fluid should be put into a red-top tube, left in the sterile syringe, and/or placed in an aerobic culturette. There are advocates of putting fluid in blood culture media to improve the chances for bacterial growth. The laboratory should be contacted for their recommendations. In many smaller animals, only one or two drops of joint fluid can be obtained. In these cases, immediate preparation of direct smears is the critical component of sample management (refer to Chapter 1). Regardless of the amount of fluid collected, it usually is advantageous to make direct smears immediately to best preserve cell morphology. These slides should not be refrigerated before staining.
Appearance and Viscosity
Normal joint fluid is typically present in small amounts (<0.5 mL) and is clear to straw-colored (Fig. 13-1A). Red-tinged fluid indicates hemorrhage or peripheral blood contamination. True hemorrhage will be uniformly discolored throughout aspiration, whereas peripheral blood contamination often occurs at the end of aspiration. This may appear as a red tail or wisp in the fluid. The fluid should be viscous as evident by stringiness when suspended between fingertips, touched by an applicator stick, or expelled from the syringe (see Fig. 13-1A). The fluid viscosity is related to the concentration and quality of hyaluronic acid. Normal synovial fluid has good viscosity and demonstrates thixotropy (see above).
Healthy synovial fluid should be viscous due to production of mucin. The mucin clot test is done to semiquantitatively assess the amount and/or degree of polymerization of hyaluronic acid in the joint fluid. Since EDTA interferes with this test, heparin can be used if an anticoagulant is required before performing this test. One to two drops of undiluted joint fluid are added to four to eight drops of 2% acetic acid. In a sample with normal hyaluronic acid concentration and quality, a thick, ropy clot will form (Fig. 13-1B). As the amount and/or quality of hyaluronic acid decreases in various forms of joint disease, the mucin clot is less well formed. This test is typically interpreted as good, fair, or poor. Normal joints have good mucin clot results.
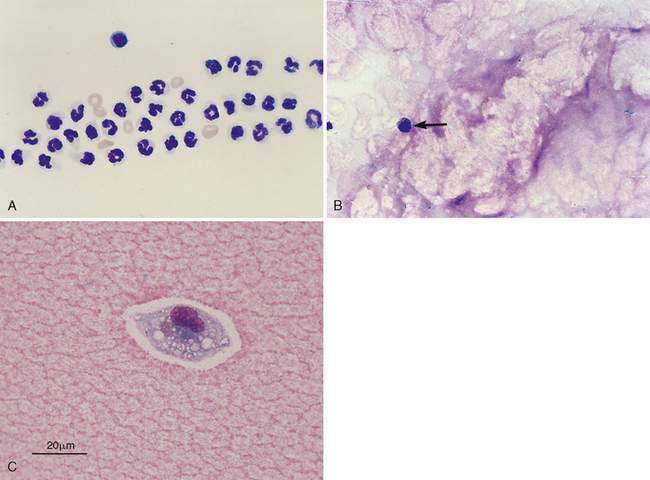
The direct smear of the synovial fluid should also be evaluated for presence of windrowing. In a viscous sample, the cells will often line up in rows or windrow (Fig. 13-2A). Mucinous material can be identified in the background of the direct smears as eosinophilic granular material (Fig. 13-2B&C) or sometimes as proteinaceous crescents.
Cell Counts and Differential
Cell counts and the differential count are done by routine methods. If enough fluid is present, cell counts can be made using a hemocytometer. Some reference laboratories use automated cell counters for cell enumeration. Automated cell counters tend to give a higher cell count than the hemocytometer; however, the difference is not usually great enough to affect the clinical interpretation. The cells may occur in clumps and accurate assessment of cell numbers may be difficult. In an effort to minimize cell clumping, hyaluronidase can be added to the synovial fluid. Various methods have been described. The easiest procedure is to add a small amount of hyaluronidase powder (amount adherent to an applicator stick) directly into the sample tube, which may result in more accurate cell counts. If only slides are prepared, cell numbers can be roughly estimated by counting the number of cells per low-power field (10×) and multiplying the count by 100 to give an approximate number per μl. However estimates from smears are less accurate and tend to be higher than counts from automated counters. Normal joints will have low nucleated cell numbers, usually fewer than 3000 cells/μl in the dog and 1000 cells/μl in the cat (Pacchiana et al., 2004), although more typically the count is fewer than 500 cells/μl in both species. These counts may vary slightly based on breed, age, body weight, and joint sampled. Consequently, only 1 to 2 cells per high-power field (40×) will be observed depending on the thickness of the direct smear (see Fig. 13-2B). Gibson et al. (1999) demonstrated the variability in performing these estimates by a group of clinicians on synovial fluid. Cells commonly observed in synovial fluid include lymphocytes, macrophages (clasmatocytes), neutrophils, and, occasionally, synovial lining cells that produce glycosaminoglycans. Neutrophils typically account for less than 5% to 10% of nucleated cells in normal joints. If fluid is obtained, both direct smears and concentrated preparations can be evaluated. If available, a cytocentrifuge is useful in preparing concentrated preparations. Concentrated preparations can also be prepared by centrifuging the fluid, pouring of the supernatant, and resuspending the fluid in one or two drops of supernatant. Smears can then be prepared from this concentrated preparation. Concentrated preparations are useful in synovial fluid particularly if the cell count is low (<500 cells/μl).
Protein Concentration
Protein concentration is often measured by refractometry, which usually provides a value that is useful for routine clinical classification and interpretation of the synovial fluid. The most accurate measurement of protein requires chemical methods. Normal synovial fluid generally has a low protein concentration (<2.5 g/dl) or commonly between 1.5 to 3.0 g/dl (MacWilliams and Friedrichs, 2003). Protein concentration will increase with inflammatory disease. False increases in protein can occur with EDTA, especially if a short sample is submitted or if the patient has received an intra-articular injection.
Classification of Joint Disease
The primary goal in synovial fluid evaluation is to distinguish inflammatory joint disease from degenerative joint disease (see Table 13-1). Other types of joint disease that may be distinguished include hemarthrosis and neoplastic disease. Further defining the disease process, as noted above, requires integrating the synovial fluid findings with other historical, physical, and laboratory findings including imaging techniques. It is important to note that synovial fluid analysis alone rarely differentiates or identifies the specific cause from among the multiple etiologic factors involved in inflammatory and noninflammatory joint diseases.
Inflammatory Joint Disease
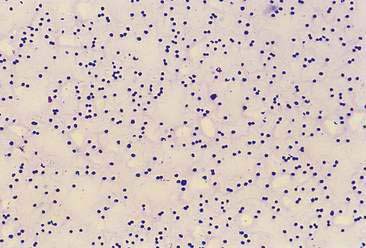
Inflammatory joint disease is characterized by finding increased numbers of white blood cells, particularly neutrophils (Fig. 13-3), in the joint fluid. Absolute numbers of segmented neutrophils are often moderately to markedly increased. However, the inflammatory process appears to cytologically wax and wane with time and, if polyarticular, involve other joints with varying intensity. Consequently, repeating joint sampling and, more importantly, sampling multiple joints, even if not clinically affected, has diagnostic value. A key point is that inflammatory joint disease has both infectious and noninfectious causes.
Infectious Arthritis
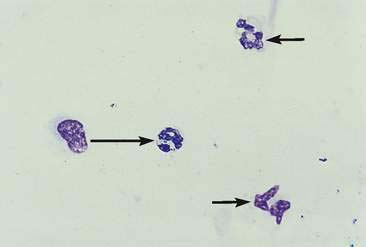
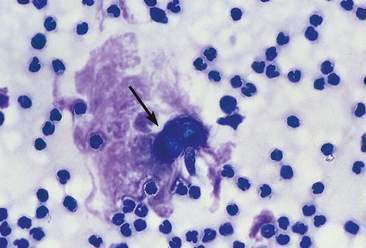
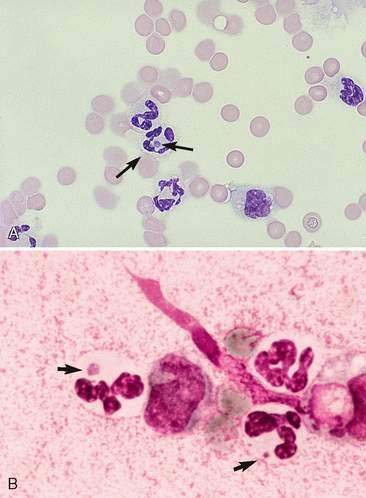
Some cases of joint disease are caused by bacterial (Fig. 13-4) or fungal infection (Fig. 13-5). In general, septic joints have very high cell counts. In most cases, the cells are primarily segmented neutrophils. It is important to evaluate the condition of the neutrophils. Degenerative or karyolytic neutrophils are more commonly observed with septic joints. Degenerate neutrophils have a pale, swollen nucleus with some loss of nuclear segmentation. However, often the majority of the neutrophils will appear nondegenerative in septic arthritis. In one study, Staphylococcus sp. was the most common bacterial agent isolated in septic joints (Marchevsky and Read, 1999). Organisms may gain access to joints either hematogenously or via direct inoculation. In addition, there may be infection elsewhere in the body (e.g., endocarditis) with immune complex deposition in the synovial tissue and resultant nonseptic inflammation in the fluid. Bacterial and fungal arthritis most commonly present with solitary joint involvement but on occasion may have multiple joint involvement, especially in young animals. Because infectious and noninfectious arthritis can have a similar presentation, it may be advisable to culture inflamed joints, keeping in mind that a negative culture does not rule out infection as the microorganisms are sometimes limited to the synovial lining tissue. Other types of organisms that have been implicated as causative agents of joint disease include mycoplasma, bacterial L-forms, spirochetes (Borrelia burgdorferi), protozoa (Leishmania donovani), viruses (calicivirus, coronavirus), and rickettsia/anaplasma such as Erlichia canis, Erlichia ewingii (Fig. 13-6A&B), Anaplasma phagocytophilum, formerly Erlichia equi, and Rickettsia rickettsi (Santos et al., 2006; Harvey and Raskin, 2004). In one study of E. ewingii joint infection cases in which the diagnosis was confirmed by polymerase chain reaction testing of peripheral blood, nucleated cell counts ranged from 16,000 to 125,000/μl with 63% to 95% neutrophils (Goodman et al., 2003).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree