CHAPTER 16 Endocrine System
The endocrine system consists of the thyroid, parathyroid, adrenal cortex, and pancreatic islet cells. These highly integrated, highly vascularized glands have sinusoids that are closely associated with secretory parenchymal cells from which hormones are produced. Also included within the endocrine system are paraganglionic cells, which are neuroendocrine cells that synthesize and secrete both catecholamines and other regulatory peptides. The neuroendocrine cells involve those of the adrenal medulla and extra-adrenal sites that are derived from neuroectoderm. The extra-adrenal paraganglionic cells include the aortic and carotid bodies, which have chemoreceptor activity in the blood gas regulation, as well as others found in the gastrointestinal tract and lung. Embryologically, neuroendocrine cells are present in gastrointestinal tissue as well as the tracheobronchial tree and liver (Hamilton et al., 1999).
THYROID TUMORS
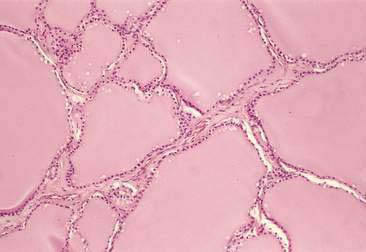
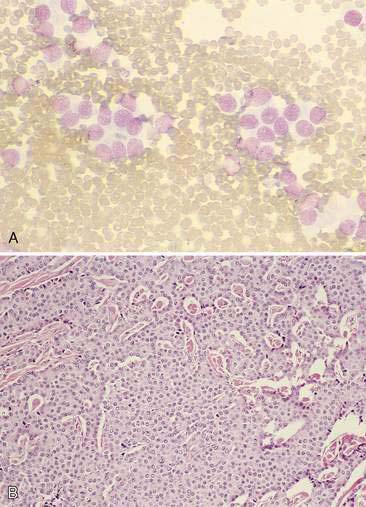
The thyroid is composed of variably sized follicles lined by simple epithelium. Squamous to low cuboidal epithelium is present in the resting stage and cuboidal to columnar epithelium is found in the active stage (Fig. 16-1). The follicular colloid contains the thyroglobulin, from which thyroid hormone is produced. Follicles are separated by delicate fibrous septa with an abundant vascular supply. The active follicle has vacuoles adjacent to the epithelium as evidence of the endocytosis of thyroglobulin.
Thyroid tumors occur most frequently in the dog, cat, and horse (Capen, 2002). They often present clinically as a subcutaneous mass located on the neck, usually lateral to the trachea, or near the thoracic inlet. Ectopic thyroid tumors may occasionally be found in the cranial thoracic cavity, at the base of the heart, or even in the oral cavity at the base of the tongue (Lantz and Salisbury, 1989). There is marked species variation regarding the biologic behavior of thyroid tumors. Therefore, the features of these lesions will be described as they relate to the dog and the cat.
Canine Thyroid Tumors
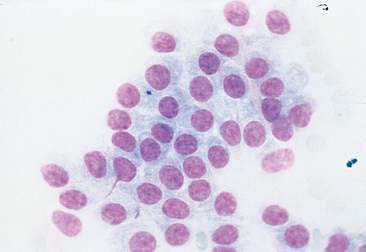
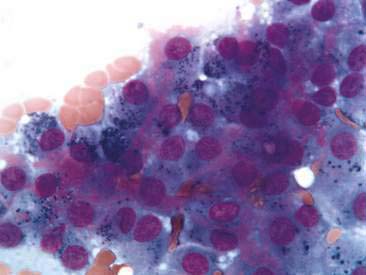
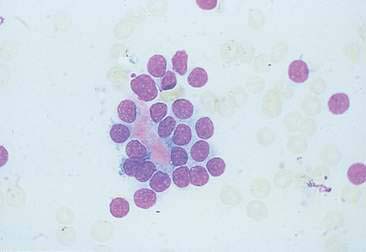
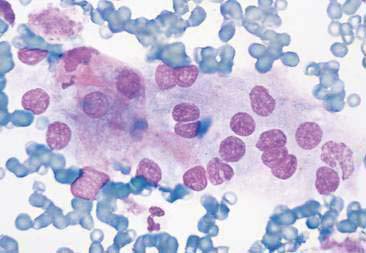
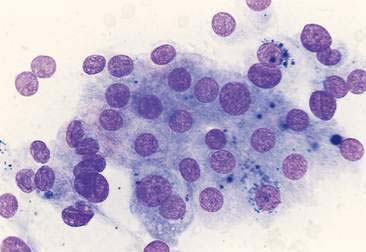
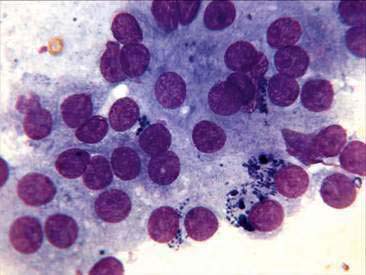
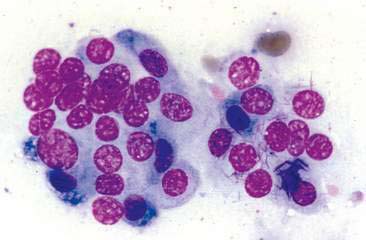
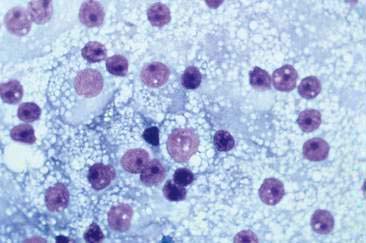
Thyroid tumors represent from 1.2% to 3.7% of all canine tumors (Harari et al., 1986). No sex predilection has been established; however, a breed predilection has been suggested for Boxers, Beagles, and Golden Retrievers (Harari et al., 1986). Approximately 50% to 70% of the thyroid tumors identified clinically in the dog are carcinomas (Bailey and Page, 2007). Aspirates from thyroid tumors, particularly carcinomas, may contain a large amount of blood contamination (Harari et al., 1986). Clusters or sheets of epithelial cells are typically seen scattered throughout the preparations. These clumps will appear as free nuclei embedded in a background of pale-blue cytoplasm, with infrequent appearance of cytoplasmic membranes or borders (Fig. 16-2). Dark-blue to black pigment is sometimes seen in the cytoplasm of epithelial cells (Fig. 16-3). Although not definitively identified as such, this pigment is thought to represent tyrosine-containing granules (Maddux and Shull, 1989). Amorphous pink material representing colloid may be associated with some clusters (Figs. 16-3 and 16-4). Colloid and/or pigmented granules, along with the naked nuclei appearance of the cells, are used to definitively identify the tissue as thyroid in origin.
The nuclei of most tumors are round to oval with minimal anaplastic features. Most thyroid tumors, even adenocarcinomas, will be composed of a fairly uniform population of cells, displaying few if any criteria of malignancy (see Figs. 16-2 to 16-4). There may be mild to moderate anisokaryosis (Fig. 16-5A) and small, indistinct nucleoli may occasionally be seen in some tumors. However, as previously mentioned, approximately 90% to 95% of the canine thyroid tumors are adenocarcinomas. Therefore, any time a canine tumor is identified as thyroid in origin it should be regarded as a probable carcinoma until histopathologic confirmation is obtained.
The biologic behavior of canine thyroid tumors is well characterized. Thyroid adenocarcinomas are invasive and will metastasize if given sufficient time. The prognosis and the potential for metastasis may depend on tumor size. In one study, 14% of dogs with tumor volumes less than 20 cm3 had evidence of metastasis, whereas a metastatic rate of 75% to 100% was seen in dogs with tumor volumes between 21 and 100 cm3 (Leav et al., 1983). The earliest and most frequent site of metastasis is the lungs, resulting from invasion of tumor cells into the thyroid or jugular vein (Capen, 2002). When possible, surgical resection is the treatment of choice; however, carcinomas are rapidly invasive and may involve vital structures such as the jugular vein, carotid artery, and esophagus. In dogs, hypersecretion of thyroid hormones in association with thyroid tumors may involve 10% of cases (Bailey and Page, 2007).
Feline Thyroid Tumors
Thyroid tumors in the cat are cytologically identical to those seen in the dog (Figs. 16-5 to 16-8). However, unlike in the dog, the vast majority of tumors in the cat are benign adenomas, also known as adenomatous hyperplasia. Both thyroid glands are involved in about 70% of the cases (Peterson et al., 1983). Functional adenocarcinomas occur in only 1% to 2% of cats presenting with clinical signs of hyperthyroidism (Turrel et al., 1988). In the cat, if cytologic preparations contain a very uniform population of nuclei with no criteria for malignancy, the thyroid tumor is considered most likely benign. It is not possible to cytologically differentiate between adenomas and adenocarcinomas. However, histologic evaluation of capsular or lymphatic invasion is often required to distinguish adenomas from adenocarcinomas (Capen, 2002; Turrel et al., 1988).
Unlike the canine, most thyroid adenomas in the cat actively secrete thyroid hormones. Adenomas are usually well encapsulated and the prognosis is excellent with surgical removal. If bilateral thyroidectomy is performed, the patient must be monitored for signs of hypothyroidism or hypocalcemia resulting from removal of the parathyroid glands (Bailey and Page, 2007). Adenocarcinomas are locally invasive and often metastasize to regional lymph nodes. Metastatic disease has been reported in up to 71% of cats with adenocarcinomas (Turrel et al., 1988).
PARATHYROID TUMORS
Parathyroid tumors are uncommon neoplasms in domestic animals. Most reported cases involve dogs (Berger and Feldman, 1987; DeVries et al., 1993) or cats (den Hertog et al., 1997; Kallet et al., 1991). There may be a breed predisposition in the Keeshond (Berger and Feldman, 1987). The tumors are usually recognized in older animals—for example, in dogs 7 years of age or older and cats 8 years of age or older.
The most frequently reported parathyroid tumor is the adenoma of the parathyroid chief cells. Parathyroid carcinomas are rare, but have been diagnosed in older dogs and cats (Capen, 2002; Kallet et al., 1991; Marquez et al., 1995). Cytologic evaluation of parathyroid tumors in the dog is usually performed on surgically removed specimens since tumors are usually too small to be detected by cervical palpation. However, in one report, four of seven cats with primary hyperparathyroidism had palpable cervical masses (Kallet et al., 1991). In some cases, cytologic evaluation has been useful in making a diagnosis (den Hertog et al., 1997). Chief cells aspirated from parathyroid adenomas have a typical naked nuclei appearance on cytologic preparations. Cells appear as free nuclei in a lightly eosinophilic background of cytoplasm. In addition, some parathyroid tumors contain needle-like, eosinophilic, cytoplasmic inclusions (Fig. 16-9). The composition of these inclusions and the frequency at which they can be found in parathyroid tumors are unknown. Nuclei are round to oval and fairly uniform in size and shape. Parathyroid adenocarcinomas typically are larger than adenomas; however, they may appear similar cytologically (see Fig. 16-9). The diagnosis of adenocarcinoma is made when there is histologic or gross evidence of capsular invasion, invasion into surrounding structures, or metastasis to regional lymph or lungs (Capen, 2002).
Since parathyroid tumors, especially adenomas, are often not palpable, the presurgical diagnosis frequently relies on recognition of clinical signs and characteristic laboratory findings. The vast majority of parathyroid tumors actively secrete inappropriate amounts of parathormone, and most cases are presented for clinical signs associated with increased hormonal activity. Although some species variation may exist with regard to the frequency of observance of specific clinical signs, similarities in clinical and laboratory findings exist in all species. Commonly reported abnormalities include hypercalcemia (the most common finding), polydipsia and polyuria, muscle weakness, skeletal abnormalities, and cystic calculi (DeVries et al., 1993; Bailey and Page, 2007 Marquez et al., 1995).
Surgical exploration of the cervical area is warranted if laboratory and clinical findings establish a diagnosis of primary hyperparathyroidism. Parathyroid adenomas are well encapsulated and can be surgically removed by blunt dissection. Patients must be monitored closely for the rapid development of postsurgical hypocalcemia (Bailey and Page, 2007). The long-term prognosis for patients with parathyroid adenomas is good.
TUMORS OF THE ENDOCRINE PANCREAS
The most frequently diagnosed tumor of endocrine pancreas is the insulinoma, a tumor of the beta islet cells (β-cells). These lesions have been referred to as insulinomas, insulin-producing pancreatic tumors, insulinproducing islet cell tumors, islet cell tumors, and β-cell tumors. They most commonly occur in dogs, generally large breeds over 5 years of age (Capen, 2002). Commonly affected breeds include Boxers, German Shepherds, Irish Setters, Poodles, Fox Terriers, Collies, and Labrador Retrievers. These tumors have also been identified with some frequency in the ferret and rarely in the cat (Hawks et al., 1992; Capen, 2002).
The cytologic appearance of insulinomas is typical of other endocrine tumors, with most preparations being fairly cellular containing mostly naked nuclei embedded in a background of lightly basophilic cytoplasm. In many instances, the cytoplasm of the cells contains numerous, small, punctate, clear vacuoles (Fig. 16-10). There may be a mild to moderate anisokaryosis and nuclei may contain a single prominent nucleolus, typical of some endocrine tumors. Although most β-cell tumors in the dog are carcinomas, nuclear features of malignancy are inconsistently seen, and as with other endocrine tumors, it is often difficult to predict the biologic behavior of these lesions based on the histologic or cytologic characteristics of the cells (Capen, 2002). Therefore, some pathologists prefer to identify these lesions as islet cell tumors unless there is evidence of invasion into surrounding structures, lymphatics, or metastatic disease. If the criteria for malignancy are met, a diagnosis of adenocarcinoma can reliably be made; however, the lack of anaplastic features cannot be used to predict the biologic behavior. Even small tumors composed of welldifferentiated β-cells have been known to metastasize (Capen, 2002).
The biologic behavior of these lesions is well characterized. Unlike in humans, in whom 90% of the pancreatic islet cell tumors are adenomas, most islet cell tumors in the dog are adenocarcinomas (Capen, 2002). Metastasis is via lymphatics with involvement of regional lymph nodes and liver in about 50% of the cases (Bailey and Page, 2007). Metastatic disease has been documented in other sites as well. Most tumors actively secrete inappropriate amounts of insulin, resulting in profound hypoglycemia. Because of the low blood glucose, patients often present with neuromuscular signs such as seizures, hind-limb weakness, ataxia, muscle tremors, and generalized weakness (Bailey and Page, 2007). Although not exclusively associated with insulinomas, most dogs with the disease have Whipple’s triad, which consists of (1) clinical signs associated with hypoglycemia, (2) fasting blood glucose less than 40 mg/dl, and (3) alleviation of clinical signs by the administration of dextrose.
A tentative diagnosis of β-cell tumor can be made by demonstrating profound hypoglycemia and an abnormal insulin-to-glucose ratio. Confirmation can be made by exploratory celiotomy or ultrasound-guided, fine-needle aspiration if the lesion is large enough. One report suggests that once a tentative diagnosis is made, exploratory celiotomy and partial pancreatectomy are indicated in dogs since surgery significantly increases the mean survival time from 74 days (medical or dietary management) to 381 days (surgery plus medical or dietary management) (Tobin et al., 1999).