7 Urinary Disorders
Differentiation Of Polyuria-Polydipsia, Dysuria, And Incontinence
Polyuria-Polydipsia
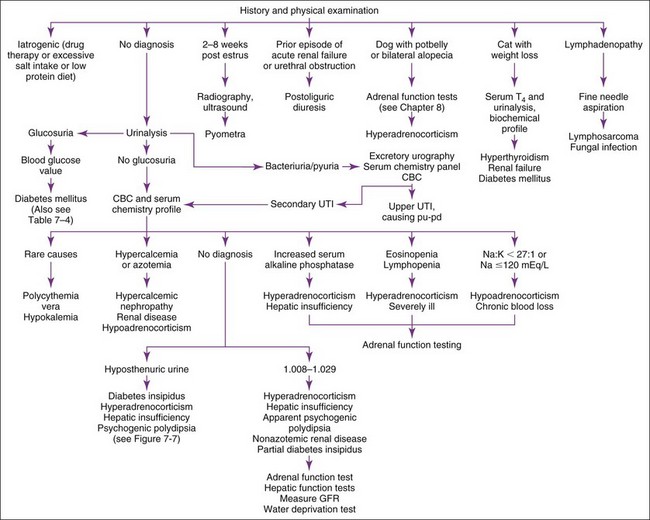
Pu-pd has many possible causes (Table 7-1). History and physical examination are crucial in evaluating patients with pu-pd (Figure 7-1). Iatrogenic causes must be sought from the history (e.g., diuretics, glucocorticoids, anticonvulsants, high-salt or very–low-protein diets, excessive thyroid supplementation). Aminoglycosides tend to produce polyuric, acute renal failure. Glucocorticoids can cause pu-pd, even when administered rectally or topically. Pyometra is usually suggested by history (2 months post-estrus) or physical examination findings (enlarged uterus, vaginal discharge). If the clinician is unsure whether pyometra is likely, a complete blood count (CBC) and abdominal imaging are usually definitive (neutrophilic leukocytosis with left shift and enlarged uterus). Weight loss plus pu-pd in a cat suggests hyperthyroidism, renal failure, or diabetes mellitus (hyperthyroidism is rare in dogs). Enlarged thyroid glands often may be palpated in the neck in hyperthyroid patients. Feline kidneys are usually palpable; size and contour should be assessed. Postoliguric diuresis is usually diagnosed from the history (e.g., a male cat that has undergone removal of a urethral obstruction). Pu-pd is the most common presenting complaint of owners of hyperadrenal dogs; most affected dogs have a pendulous abdomen, hepatomegaly, alopecia, or a combination thereof on physical examination. Such dogs should undergo adrenal function testing (see Chapter 8). Recent onset of cataracts suggests diabetes mellitus. Peripheral lymphadenopathy suggests lymphoma causing hypercalcemia. Other neoplasms (e.g., anal sac apocrine gland adenocarcinomas) and certain infectious diseases (e.g., systemic mycoses) may also cause hypercalcemia.
TABLE 7-1 CAUSES OF POLYURIA-POLYDIPSIA (PU-PD) IN DOGS AND CATS
| CAUSES | REMARKS |
|---|---|
| Iatrogenic; drugs (e.g., diuretics, corticosteroids, thyroxine, anticonvulsants, aminoglycosides, or amphotericin B); salty or very-low-protein diets | History is informative |
| BUN should be low with low-protein diets | |
| Renal disease | Urine specific gravity should be persistently 1.008–1.029 (dogs) |
| May be azotemic or nonazotemic | |
| For nonazotemic renal failure, measurement of GFR is useful for diagnosis of renal dysfunction as the cause | |
| Upper urinary tract infection | May be hyposthenuric |
| Excretory urography is diagnostic test of choice, but ultrasound may be helpful | |
| Fanconi’s syndrome | Usually nonazotemic, hyperchloremic acidosis, glucosuric, and aminoaciduric |
| Diabetes mellitus | Hyperglycemic |
| Note: Cats are prone to stress-induced hyperglycemia; urine glucose measured at the same time is usually (but not always) negative | |
| Central diabetes insipidus | Hyposthenuric when euhydrated (see Figure 7-8) |
| Nephrogenic diabetes insipidus | Hyposthenuric when euhydrated |
| May be primary (congenital), idiopathic, or secondary (pyelonephritis, hyperadrenocorticism, hypercalcemia, hypokalemia, pyometra, prostatic abscessation, E. coli septicemia, hypoadrenocorticism) | |
| Hyperadrenocorticism | Isosthenuric, hyposthenuric, or concentrated urine |
| Common cause of pu-pd in old dogs, rare in cats | |
| Hypoadrenocorticism | Pu-pd occurs in approximately 20% of patients; may closely resemble renal failure but differentiated by absence of a stress leukogram and presence of hyperkalemia despite polyuria; ACTH response test is necessary for confirmation |
| Hypercalcemia | Isosthenuric or hyposthenuric, azotemic or nonazotemic |
| Hepatic insufficiency | Isosthenuric, hyposthenuric, or concentrated; may resemble hyperadrenocorticism (hepatic enzymes may be increased), but can also have normal ALT and SAP |
| Hyperthyroidism | Primarily in older cats but may be iatrogenic because of supplementation |
| Hyponatremia | Loss of sodium from any cause |
| May cause isosthenuria whenever <120 mEq/L | |
| Post–urethral obstruction | Occurs occasionally after removal of a urethral obstruction that has resulted in uremia |
| Hypokalemia | Must be persistent and severe to cause polyuria |
| Polycythemia vera | Rare |
| Apparent psychogenic polydipsia | Concentrate urine in response to water deprivation (see Figure 7-8) |
| Acromegaly | Rare |
ACTH, Adrenocorticotropic hormone; ALT, alanine aminotransferase; BUN, blood urea nitrogen; GFR, glomerular filtration rate; SAP, serum alkaline phosphatase.
If the cause is not obvious, a urinalysis, CBC, and biochemical profile are the next steps (see Figure 7-1). Urinary tract infections (UTIs) may be secondary to hyperadrenocorticism or diabetes mellitus, but renal infection (i.e., bacterial pyelonephritis) can cause pu-pd. Neutrophilic leukocytosis, azotemia, white blood cell (WBC) casts, renal pain, or hyposthenuria may occur with pyelonephritis. Pyelonephritis can be difficult to diagnose; excretory urography is the diagnostic method of choice (although abnormalities may also be found with ultrasonography if the renal pelvis can be imaged). Sometimes a presumptive diagnosis can only be made by the necessity for long-term antibiotic therapy after failure of short-term therapy. Clinicopathologic screening often reveals changes indicative of the cause of pu-pd (e.g., renal failure, hyperadrenocorticism, hepatic insufficiency, hypercalcemia, diabetes mellitus) (see Figure 7-1). Serum thyroxine determinations are always indicated in older cats (i.e., >10 years old).
Many dogs with hyperadrenocorticism are obviously cushingoid on physical examination (i.e., cutaneous abnormalities, potbellied appearance, and hepatomegaly). Common CBC and serum chemistry profile changes include lymphopenia, eosinopenia, and increased serum alkaline phosphatase (SAP), alanine aminotransferase (ALT), and serum cholesterol. Approximately 40% to 50% of hyperadrenal dogs have UTI. Bacteriuria is often the only abnormality on urinalysis (i.e., no hematuria or pyuria). Adrenal function tests (see Chapter 8) or hepatic biopsy may be necessary to distinguish hyperadrenocorticism from primary hepatic disease.
Renal failure, hypercalcemic nephropathy, and hypoadrenocorticism can resemble each other. The first two usually produce pu-pd, whereas the last causes it in 15% to 25% of affected dogs. Each may have azotemia, decreased renal concentrating ability (e.g., specific gravity 1.012 to 1.029), and hypercalcemia (10% to 15% of renal failure patients and 30% of hypoadrenal dogs). Most hypoadrenal patients have a serum Na : K of less than or equal to 27 : 1 with hyponatremia, hyperkalemia, or both. Classically, no stress leukogram exists despite the animal being ill. An adrenocorticotropic hormone (ACTH) stimulation test (see Chapter 8) is needed to confirm the diagnosis, because other disorders may cause similar changes. Most azotemic renal failure patients are hyperphosphatemic and only mildly hypercalcemic (i.e., <13 mg/dl), whereas most animals with hypercalcemia of nonrenal origin are normophosphatemic or mildly hypophosphatemic and may be markedly hypercalcemic. Nevertheless, distinguishing whether hypercalcemia is the cause or consequence of renal failure can be difficult if persistent hypercalcemia has produced renal damage plus hyperphosphatemia (especially when hypercalcemia is mild [11.5 to 14 mg/dl]). Such patients need a thorough search for neoplasia and require measurement of ionized calcium and parathyroid hormone (PTH) concentrations (see Chapter 8). Most dogs with hypoadrenocorticism and primary renal failure have normal to decreased ionized calcium, whereas animals with primary hypercalcemic disorders (i.e., hyperparathyroidism, hypercalcemia of malignancy) have increased ionized calcium concentrations.
More extensive testing is necessary if the diagnosis is still uncertain (see Figure 7-1). Some dogs with renal failure are polyuric because of loss of functional nephron number (may occur with 67% reduction in renal function) but maintain a sufficient glomerular filtration rate (GFR) to avoid azotemia (which requires a 75% reduction in GFR). A creatinine or iohexol clearance test is a noninvasive way to identify these patients. Water deprivation and antidiuretic hormone (ADH) response testing can be useful if the patient is not azotemic. Elimination of other causes also allows a reasonable tentative diagnosis.
Dysuria
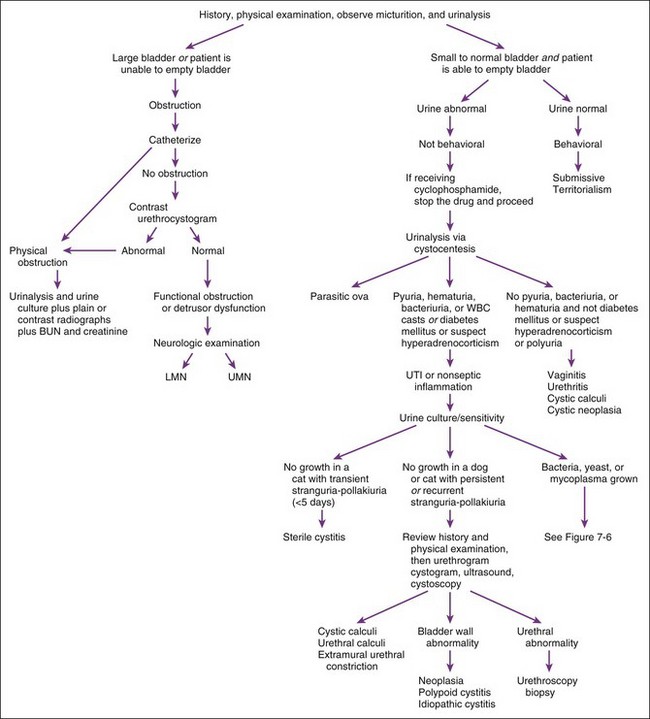
Alterations in behavior associated with urination (other than polyuria) generally suggest disorders affecting the urinary bladder, urethra, or both (Figure 7-2). Irritative or inflammatory (septic or nonseptic) lesions that do not impede urine flow typically cause animals to urinate small volumes and to urinate more often, perhaps with apparent discomfort (i.e., dysuria). Urethral obstruction causes animals to make repeated voiding efforts that are either unproductive (i.e., complete obstruction) or somewhat productive but the bladder cannot be emptied (i.e., partial obstruction). Some animals with urethral obstruction have urinary incontinence. Such paradoxical incontinence occurs when accumulated urine is forced past an obstruction by markedly increased intravesicular pressure.
Incontinence
After the history and physical examination, the incontinence should be classified as neurogenic (associated with other neurologic problems) or not (unassociated with other neurologic signs). When the neurologic examination is abnormal, the lesion should be localized and further diagnostic tests pursued to identify the cause. Even when the neurologic examination is normal, a local neuromuscular cause (such as detrusor dysfunction) may be the cause of incontinence. Incontinence in animals with a normal neurologic examination is best approached by subdividing it into two categories on the basis of the physical examination: a distended bladder with inability to completely empty or a normal bladder with ability to void (Table 7-2).
TABLE 7-2 CAUSES OF INCONTINENCE AND DIAGNOSTIC TESTS
| CATEGORY | RULE OUTS | POSSIBLE DIAGNOSTIC TESTS |
|---|---|---|
| ASSOCIATED WITH OTHER NEUROLOGIC SIGNS | Cerebral lesions | MRI, CT, CSF |
| Brainstem lesions | MRI, CT, CSF | |
| Spinal cord lesions (cervical, thoracic, lumbar) | MRI, CT, spinal radiographs, myelography, CSF, EMG | |
| Lesions of the sacral spinal cord, roots, pelvic or pudendal nerves | MRI, CT, epidurogram, CSF, EMG | |
| UNASSOCIATED WITH OTHER NEUROLOGIC SIGNS; Inability to Empty the Bladder | Urethral obstruction | Palpation per rectum, radiography, urethrography, urethroscopy |
| Mass in the bladder neck area | Palpation, bladder ultrasound, cystourethrography, cystoscopy, aspiration, biopsy | |
| Detrusor-urethra dyssynergia | Diagnosis by exclusion | |
| Detrusor dysfunction | History, CMG | |
| UNASSOCIATED WITH OTHER NEUROLOGIC SIGNS; Ability to Empty the Bladder | Urethral incompetence | History, urinalysis, urine culture, urethral pressure profile |
| Ectopic ureter(s) | History, vaginourethrogram, excretory urography, urethrocystoscopy | |
| Mass in the bladder neck area | Palpation, bladder ultrasound, cystourethrography, cystoscopy, aspiration, biopsy | |
| Patent urachus | Physical examination | |
| Reduced bladder capacity | Bladder ultrasound, cystourethrography, CMG, cystoscopy, aspiration, biopsy |
CMG, Cystometrogram; CSF, cerebrospinal fluid; CT, computed tomography; EMG, electromyography; MRI, magnetic resonance imaging.
Urinalysis
Analysis
Next, 3 to 5 ml of urine should be centrifuged at approximately 2000 rpm for 5 minutes. Standardization of volume, speed, and duration of centrifugation is important for comparing results from different samples. If the uncentrifuged urine sample was visibly hemorrhagic or very turbid, the clinician or technician should repeat the dipstick analysis and the specific gravity on the supernatant. Next, most of the supernatant should be decanted, leaving approximately 0.5 ml in the tube, and the sediment in the remaining supernatant should be resuspended. The clinician or technician should transfer a drop of the reconstituted sediment to a microscope slide and place a coverslip over it. The intensity of the microscope light should be dimmed, and the clinician or technician should examine under low power (10×) for casts, crystals, and cells. The number of casts per low-power field (lpf) should be counted, and then the specimen should be examined under high power (40×) to identify cells and bacteria. The clinician or technician should count the number of WBCs and red blood cells (RBCs) per high-power field (hpf) and estimate the number of bacteria (i.e., trace, moderate, many). Sediment stains can be used. Visualization of bacteria is enhanced by the use of either Gram2 or modified Wright staining.49 Finally, the results should be recorded, with a notation of how urine was collected and how many milliliters of urine were spun if it was less than 3 to 5 ml.
Color and Turbidity
Analysis
Color and turbidity are analyzed by visual inspection. Normal urine is clear to slightly turbid and light yellow to amber. Dilute urine tends to be colorless, and concentrated urine is dark yellow. Different colors and their significance are listed in Table 7-3. Significant disease may exist even if the urine is normal in color and turbidity. If urine discoloration is noted, one should review the patient’s history for drug administration and carefully examine the urine sediment. Hematuria, hemoglobinuria, and bilirubinuria are the most common causes of discolored urine. Pyuria, hematuria, crystalluria, and lipiduria are common causes of increased turbidity.
TABLE 7-3 POTENTIAL CAUSES OF DISCOLORED URINE IN DOGS AND CATS
| URINE COLOR | CAUSES |
|---|---|
| Dark yellow | Concentrated urine |
| Pale yellow | Normal urochromes, urobilin |
| Yellow-orange | Bilirubin |
| Fluorescein | |
| Concentrated urine | |
| Phenazopyridine | |
| Green-blue | Methylene blue |
| Dithiazanine | |
| Biliverdin | |
| Brown-black | Bile pigments |
| Myoglobin | |
| Methemoglobin | |
| Yellow-brown | Bile pigments |
| Red | Hemoglobin |
| RBCs | |
| Myoglobin | |
| Dyes | |
| Phenazopyridine | |
| Phenolsulfonphthalein | |
| Milky | Pyuria |
| Lipiduria | |
| Phosphate crystals | |
| Colorless | Dilute |
RBCs, Red blood cells.
Specific Gravity
Analysis
Specific gravity is determined with a refractometer. One should periodically check the refractometer’s calibration by verifying that distilled water gives a reading of 1.000. Storage of urine in capped containers at room or refrigeration temperature for at least 24 hours does not affect measurement of specific gravity.1 Some dipsticks have a test pad that indicates specific gravity; however, these results are inaccurate.6 Use of dipstick test pads to evaluate urine specific gravity is not recommended.
Normal Values
Specific gravity varies in normal dogs and cats, and any random specific gravity may be normal in euhydrated animals. Specific gravities less than 1.020 may be associated with polyuria evident to the owner. Specific gravity is important to assess renal function in dehydrated or azotemic animals. The urine of a dog with evident dehydration and normal renal function should have a specific gravity greater than 1.030, and a cat’s should be greater than 1.035. Puppies do not concentrate urine until they reach 4 weeks of age.17
Causes of Altered Urine Specific Gravity
A single urine specific gravity greater than 1.007 and less than 1.030 (dog) or 1.035 (cat) does not imply renal tubular dysfunction or pu-pd unless the patient is clinically dehydrated or azotemic, in which case such a specific gravity reflects abnormal renal tubular function. Otherwise, one must document failure to concentrate urine adequately during water deprivation testing to establish urine concentrating ability as abnormal. Persistently hyposthenuric or isosthenuric urine is an indication for further testing (see Figure 7-1).
Urine pH
Analysis
Urine pH is usually measured with a pH test pad on a urine reagent strip. However, results are not very accurate as compared to pH meters.29,34 Portable pH meters should be used when accurate urine pH measurements are needed. Storage of urine in capped containers at room or refrigeration temperatures for at least 24 hours does not affect urine pH as measured with a pH meter.1
Causes of Acid or Alkaline Urine
Persistently alkaline urine is an indication for complete urinalysis and urine culture. If no reason for alkaline urine is found on history, urinalysis, or urine culture, distal RTA may be considered, although it is rare. Both distal and proximal RTA cause hyperchloremic metabolic acidosis with a normal anion gap and often produce hypokalemia (see Chapter 6).
Proteinuria
Analysis
Proteinuria associated with hyperglobulinemia due to myeloma (see Chapter 12) may be caused by Bence Jones proteins (free light chains). Bence Jones proteins do not cause a positive dipstick reaction but do cause a positive result on precipitation testing. Serum and urine protein electrophoresis are indicated in such patients, in which case most of the protein is a monoclonal spike in the β or γ regions. Such a finding necessitates a search for osteolytic or lymphoproliferative lesions. Ehrlichiosis occasionally mimics myeloma (i.e., glomerulonephritis, monoclonal-like gammopathy, bone marrow plasmacytosis). A titer may be diagnostic (see Chapter 15).
A semiquantitative test (ERD-HealthScreen; Heska Corporation—see Appendix I) is available to detect low concentrations (1 to 30 mg/dl) of albumin in urine, with separate tests for dogs and cats. This test is affected by macroscopic hematuria and by inflammation indicated by pyuria. The test is indicative of any illness, not necessarily primary renal disease, and positive results increase with age.54–56 Repeatability problems have been reported in cats with this test, and the test did not always detect cats with urine protein : urine creatinine (UPC) ratios greater than 0.5.40
Normal Values
A trace of proteinuria or 1+ reaction is probably normal with a specific gravity greater than 1.012 in dogs.57 If the urine specific gravity is less than 1.012, any amount of proteinuria may be abnormal. Dipstick and sulfosalicylic acid precipitation tests had such a high level of false-positive results that a normal value was difficult to determine in cats.39,40 The dipstick and precipitation tests are not quantitatively accurate. Quantitative tests are needed to precisely determine severity of protein loss, as discussed under Urine Protein : Urine Creatinine Ratio in the next section of this chapter.
Drug Therapy That May Cause Proteinuria
Any drug that causes renal tubular or glomerular injury can cause proteinuria (Box 7-1).
Box 7-1 Selected Potentially Nephrotoxic Drugs*
Cyclophosphamide (nephrotoxicity is uncommon; sterile cystitis is more common)
Dextran (low molecular weight)
Heavy metals (i.e., gold, lead, mercury)
Radiographic contrast media (important when there is preexisting azotemia and dehydration)
Sulfonamides (uncommon if more soluble sulfonamides are used)
Causes of Proteinuria
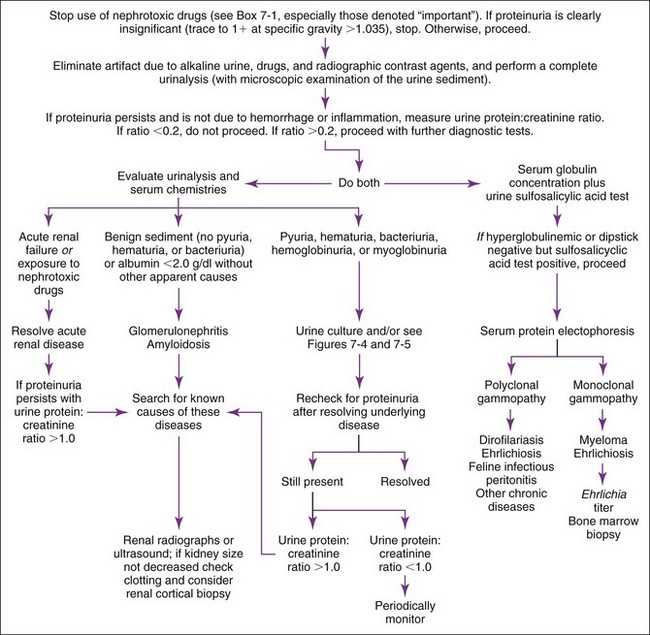
One must first decide if the proteinuria is significant or not by examining the specific gravity, as described earlier (Figure 7-3). If it is insignificant, one may ignore it unless the patient is receiving nephrotoxic drugs (e.g., aminoglycosides). Such drugs should be stopped regardless of the amount of proteinuria, because mild proteinuria may be an early sign of nephrotoxicity and impending acute renal failure. Aminoglycoside nephrotoxicity typically causes proteinuria or other urinalysis changes (e.g., isosthenuria, glucosuria, cylindruria) before azotemia.
If hemorrhage and inflammation are excluded by a normal (“quiet” or “inactive”) urine sediment and prerenal causes are unlikely, proteinuria is most likely of renal origin. Renal-origin proteinuria can be transient or persistent and due to glomerular or tubular dysfunction. One should measure serum albumin and repeat the urinalysis to determine if proteinuria is persistent. If hypoalbuminemia is present in association with marked proteinuria, one can assume that the proteinuria is significant and persistent. Transient (i.e., functional) proteinuria has many causes (e.g., strenuous exercise, fever, seizures, venous congestion of the kidneys) and is rarely significant. Persistent proteinuria is defined as abnormal proteinuria on at least three occasions, two or more weeks apart.37 Persistent proteinuria with an inactive urine sediment should prompt determination of a UPC ratio to ascertain its severity.
Urine Protein:Urine Creatinine Ratio
Advantages
Measurement of the UPC ratio is more accurate than dipstick and precipitation procedures, requires only a single random urine sample, and correlates well with 24-hour determinations. Urine samples can be collected by any method.7,25
Disadvantages
A complete urinalysis should be performed on an aliquot of the same sample to look for macroscopic hemorrhage or inflammation, which would alter assessment of the results. In dogs with abnormal proteinuria, the UPC ratio varies day-to-day especially in the lower ranges of abnormality.42 This variation can be as much as 80% at values near 0.5 and 35% at values about 12 even when the same methodology is used. A single measurement will reliably estimate the UPC ratio at values less than 4, but higher values require two to five determinations. Such variation should be taken into account when following UPC ratios in dogs and cats with chronic renal diseases. The ratio gives no information about the origin of the proteinuria; it only quantifies it.
Analysis
Normal Values
Adult dogs and cats, less than 0.2 (higher values are normal in puppies at least up to 2 weeks of age46 and in noncastrated male cats); borderline 0.2 to 0.5 (dogs), 0.2 to 0.4 (cats).
Danger Values
None. However, in azotemic and in hypertensive cats, the risk for death or euthanasia increases with increasing UPC ratio.50,51 In nonazotemic, geriatric cats, the UPC ratio was predictive of survival and of development of azotemia.33 In dogs with renal failure, a UPC value greater than 1 at initial evaluation was associated with increased risk of uremic morbidity and mortality.32 Marked loss of albumin in urine can lead to hypoalbuminemia.
Artifacts
See discussions of total protein (see Chapter 12) and of creatinine later in this chapter.
Causes of Increased UPC Ratio
Marked proteinuria (UPC ratio > 2) associated with a quiet sediment and normal serum globulins or a polyclonal gammopathy is usually the result of renal glomerular disease (i.e., glomerulonephritis, amyloidosis). One should search for causes of glomerulonephropathy: chronic parasitic disease such as heartworm disease, hepatozoonosis, or leishmaniasis; chronic inflammatory diseases such as systemic lupus erythematosus; chronic infectious diseases such as borreliosis, feline leukemia virus (FeLV) infection, feline immunodeficiency virus (FIV) infection, ehrlichiosis, pyometra, or endocarditis; neoplasia; endocrine diseases such as hyperadrenocorticism31,43 and diabetes mellitus48; and familial glomerulopathies. Immunosuppressive glucocorticoid therapy leads to mild proteinuria.47,53 If no underlying disease is identified or if proteinuria persists despite treatment for an identified disease, renal cortical biopsy should be considered to determine whether glomerulonephritis or amyloidosis is present.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree