14 Neurologic Disorders
Neuroanatomic Localization
In the coming years, neurologic examination–derived head trauma and spinal cord injury scales are likely to play an increasing role in veterinary medicine. Scales facilitate health-care professional communication, objective accounting of clinical progress, and prediction of recovery. The modified Glasgow Coma Scale has been used in dogs with head trauma and is predictive of 48-hour outcome.23 In dogs with disk-associated spinal cord injury, the modified Frankel Scale, Texas Spinal Cord Injury Scale, and 14-point pelvic limb motor score are valid means to qualify lesions severity, correlate to MRI markers of injury, and are easily performed.13 The authors strongly encourage clinicians to make standard and appropriate use of these systems to facilitate better care delivery.
Differential Diagnoses
Differential diagnosis lists are based on neuroanatomic localization, signalment, as well as onset, progression, and duration of clinical signs (Tables 14-1 and 14-2). Neoplastic diseases, disk herniation, and idiopathic epilepsy all have age and breed predilections. Vascular diseases of the CNS usually have a rapid onset and are nonprogressive, whereas CNS degenerative diseases are insidious in their onset and clinical course. Appropriate neuroanatomic localization coupled with a strong differential list will allow clinicians to formulate a diagnostic plan.
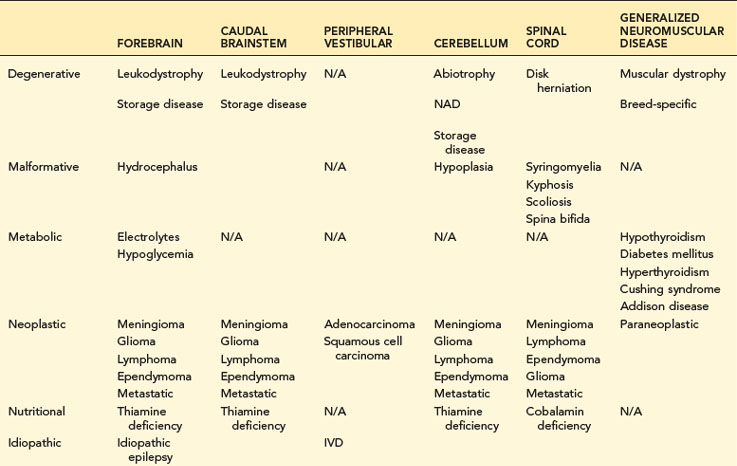
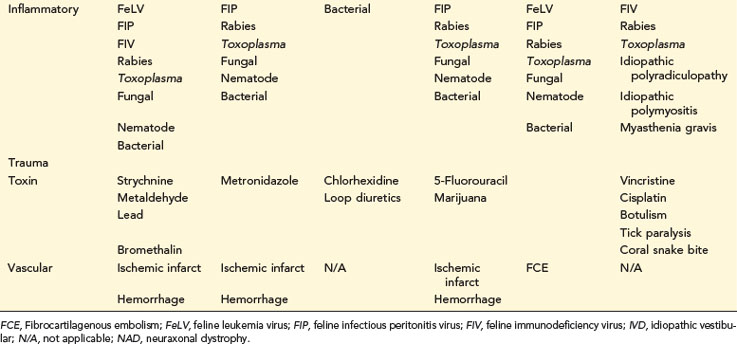
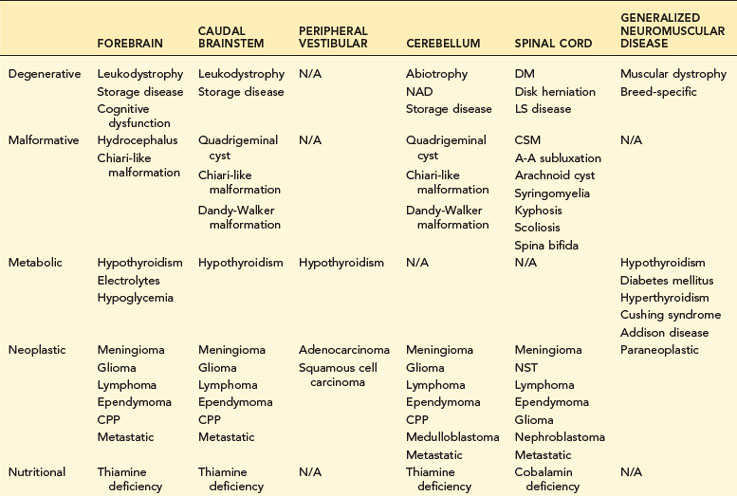
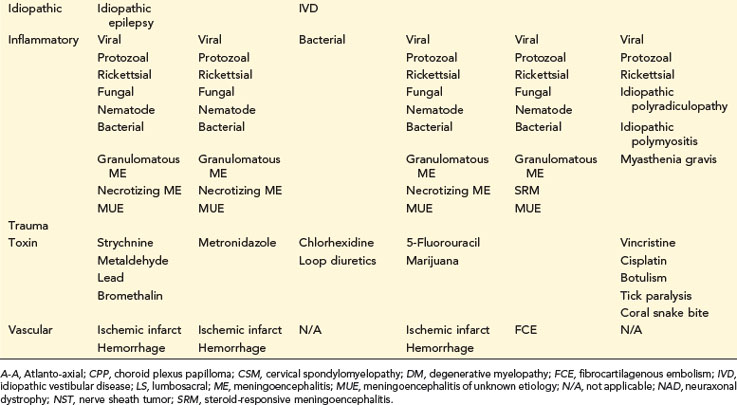
TABLE 14-1 COMMON NEUROLOGIC DISEASES IN DOGS, LISTED BY NEUROANATOMIC LOCALIZATION AND ETIOLOGIC CATEGORY
Neuroimaging
Vertebral Column Indications
Radiography, myelography, computed tomography (CT), and MRI can be used to assess the vertebral column. Radiography is probably best viewed as a cost-effective means of screening for overt structural diseases (e.g., fracture, luxation, discospondylitis, large lytic bony tumor). Diagnostic accuracy of radiographs for disk herniation, cervical spondylomyelopathy, or intraparenchymal spinal cord disease is poor. Myelography shares many of the disadvantages of radiography in that the spinal cord cannot be directly visualized and images are available for review in a limited number of planes; myelography is also associated with adverse patient events (e.g., seizure, post-myelogram myelopathy, death). CT is the best means to evaluate bony pathology and is the study of choice to define vertebral fracture or luxation. Like myelography, soft tissue detail is not ideal; therefore visualization of intraparenchymal spinal cord lesions or nonmineralized disk herniation may be challenging. MRI has revolutionized diagnosis of vertebral column diseases. Signal patterns may indicate certain pathologic processes (e.g., hemorrhage, edema), can be specific for certain etiologies, and may provide data relating to outcome. For example, each L2 vertebral length of T2-weighted hyperintensity within the spinal cord of dogs with thoracolumbar disk herniation lowers the probability of walking by 1.9-fold.14
Cerebrospinal Fluid Analysis
Common Indications
Cerebrospinal fluid (CSF) is one of the only means to assess cellular responses to nervous system disease. Routine collection is suggested in animals with CNS signs or radiculopathy. In animals with intracranial CNS disease, collection is usually performed after neuroimaging (see “Contraindications”). CSF should always be acquired prior to myelography to prevent delivery of intrathecal iodinated contrast in animals with primary CNS inflammation and to avoid post-myelographic pleocytosis from hindering CSF interpretation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree