15 Microbiology and Infectious Disease
Infectious agents may be identified directly by cytologic analysis, histopathologic evaluation, culture, viral isolation, antigen detection, or nucleic acid amplification techniques. Polymerase chain reaction (PCR) assays are the most commonly used nucleic acid amplification techniques in small animal infectious diseases. Detection of antibodies against infectious agents provides indirect evidence of prior exposure or current infection. This chapter describes methods for obtaining specimens, outlines currently used testing procedures for some of the more common infectious diseases, and discusses interpretation of results from the various procedures and tests.*
When to Suspect Bacterial/Fungal/Rickettsial Viral Agents
Finally, clinicopathologic abnormalities can suggest disease caused by infectious agents. Neutrophilic leukocytosis, particularly if a left shift or degenerative neutrophils (see Chapter 4) are also present, is consistent with an infectious cause of disease. Gram-negative sepsis is suggested by leukopenia with a degenerative left shift. Monocytosis or lymphocytosis can be induced by persistent infection with a number of intracellular agents that result in persistent infection. Examples include ehrlichiosis, toxoplasmosis, and bartonellosis. Polyclonal (e.g., multiple infectious causes) or monoclonal (e.g., usually induced by neoplasia, rarely associated with canine ehrlichiosis) gammopathies may suggest chronic immune stimulation. Neutrophils in aqueous humor, cerebrospinal fluid (CSF), synovial fluid, or urine may indicate inflammation induced by infectious agents.
Cytology
Specimen Procurement and Analysis
See Chapter 16 for discussion of cytologic techniques and cytologic conclusions.
Bacterial Diseases
Discharges from animals with suspected bacterial disease should be placed on a microscope slide, air dried, fixed, and stained with both Gram and Romanowsky-type stains (see Chapter 16). The examination is started on low power (10× magnification), with oil immersion (100×) used for inspection of bacterial morphologic features (i.e., rods, cocci) and Gram stain characteristics (i.e., Gram-positive [blue] or Gram-negative [pink]). The primary disadvantage of Gram staining is that Gram-negative bacteria may be difficult to find because background material stains pink. It is easier to find bacteria (dark-blue stain) and easier to study morphologic detail of other cells (i.e., inflammatory cells) using Romanowsky-type stains. Gram staining may be variable; organisms in body fluids may stain differently from those grown on a blood agar plate. Gram stain demonstrates the gram-positive, branching filaments of Actinomyces spp. and Nocardia spp. (see Figure 10-11). Acid-fast stains can be used for Mycobacterium spp. and to help differentiate Nocardia spp. (acid-fast) from Actinomyces spp.
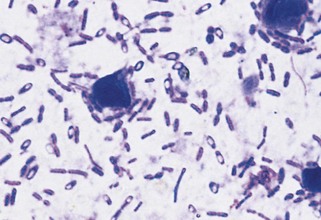
Some bacteria have characteristic morphologic features. Large rod-form bacteria containing spores found on fecal cytology of dogs or cats with diarrhea suggest Clostridium perfringens (Figure 15-1). Bipolar-staining, gram-negative coccobacilli found in aspirates of inflamed cervical lymph nodes from cats in the Southwest or West suggest Yersinia pestis. Short spirochetes found on fecal cytology of animals with diarrhea suggest but do not prove campylobacteriosis. Spirochetes found on cytology of gastric mucosa of vomiting animals suggest helicobacteriosis.
Fungal Diseases
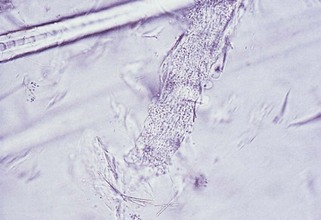
For identification of dermatophytes, hairs are plucked from the periphery of a lesion, placed on a microscope slide, and covered with 10% to 20% potassium hydroxide to clear debris. The slide is then heated (not boiled) and examined under the 10× or 40× objective to search for hyphae, spores, conidia, budding yeasts, and fungus-induced damage (e.g., swollen or broken hair shafts). The 40× objective is used to identify arthrospores (dense aggregates of spherical structures that may cover the hair shaft [Figure 15-2]). Failure to find arthrospores does not rule out dermatomycosis. Culture is more sensitive for diagnosis of dermatophytosis (see Fungal Culture).
Romanowsky-type stains (e.g., Wright) are used in preference to wet mount preparations and ink when looking for fungi other than dermatophytes (see Chapter 16). Romanowsky-type stains are also useful in identifying yeasts such as Blastomyces dermatitidis, Histoplasma capsulatum, Sporothrix schenckii, Coccidioides immitis, or Cryptococcus spp. (see Figure 11-3) in exudates, CSF, lymph node aspiration cytology, or transtracheal aspiration cytology.
Viral Diseases
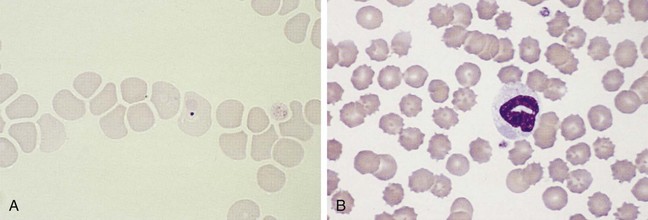
Canine distemper virus inclusions in lymphocytes, neutrophils, or erythrocytes (Figure 15-3) are diagnostic of infection but are only present transiently, so false-negative results are common. Feline herpesvirus 1 (FHV-1) infection transiently results in intranuclear inclusion bodies in epithelial cells of the conjunctiva. Rarely, FIP-inducing strains of coronavirus result in transient intracytoplasmic inclusions in circulating neutrophils.
Culture and Antimicrobial Susceptibility
Common Indications
Culture and antimicrobial susceptibility are indicated in most suspected bacterial diseases (Box 15-1), especially when clinical syndromes have been resistant to medications. Remember: Skin and mucosal surfaces have a resident microflora (Box 15-2); therefore care must be taken to avoid contamination.
Box 15-1
Bacteria Commonly Isolated from Various Sites in Infectious Disorders in Dogs and Cats
Data compiled from Greene CE, editor: Clinical microbiology and infectious diseases of the dog and cat, Philadelphia, 1998, WB Saunders.
Box 15-2
Normal Bacterial Flora at Various Sites in Dogs and Cats
Gastrointestinal Tract
Data compiled from Greene CE, editor: Clinical microbiology and infectious diseases of the dog and cat, Philadelphia, 1998, WB Saunders.
Bacterial Culture
Specimen Procurement
Body Cavities
The site of skin puncture should be prepared as for blood culture (see discussion in Cardiovascular System). If pyothorax or peritonitis seems likely but fluid cannot be aspirated, lavage (see Chapter 10) is indicated. Because mixed infections are common and pure anaerobic infections may occur, aerobic and anaerobic cultures should be performed.
Central Nervous System
Bacterial infection of the central nervous system (CNS) is uncommon. Even when infection occurs, low numbers of organisms make cytology and culture low-yield procedures. If increased numbers of neutrophils and increased protein are detected in CSF (see Chapter 14), however, aerobic and anaerobic bacterial culture and antimicrobial susceptibility testing are indicated. CSF samples should be placed in transport media and delivered to the laboratory as soon as possible. Aerobic and anaerobic bacterial culture should be performed when bacterial infection of the CNS is suspected.
Gastrointestinal Tract
Primary bacterial gastroenteritis occasionally occurs. Salmonella spp., Campylobacter spp., C. perfringens, and Escherichia coli are agents that can be involved. These organisms can also be isolated from normal animals, however. Salmonella spp. and Campylobacter spp. can cause small or mixed bowel diarrhea; C. perfringens is usually associated with large bowel diarrhea. Approximately 2 to 3 g of fresh feces should be submitted to the laboratory for optimal results. If delayed transport of feces to the laboratory is expected, the clinician should consult the laboratory for appropriate transport media. Because these organisms have special culture requirements, the laboratory must be notified of the suspected pathogen. A positive culture for C. perfringens does not prove it was the cause of disease because not all C. perfringens produce enterotoxin. Conversely, not all enterotoxin-positive animals have diarrhea. Thus culture or PCR for C. perfringens should be combined with enterotoxin measurement (See Chapter 9).
Musculoskeletal System
No normal flora exists in musculoskeletal tissues. Dogs with radiographic evidence of discospondylitis should be evaluated for Brucella canis and Bartonella spp. infections (see Diagnostic Tests for Select Bacterial Infections). Intervertebral joints can be cultured after fluoroscopically guided aspiration or when decompressive spinal surgery is required. Most cases of discospondylitis develop after hematogenous spread of bacteria from an extravertebral source. Blood and urine are commonly cultured from patients with discospondylitis; Staphylococcus spp. are commonly involved.
Dogs or cats with suppurative arthritis (with or without cytologic visualization of bacteria) should have the synovial fluid cultured for aerobes and Mycoplasma spp. (see Chapter 10). Likelihood of positive culture results increases if the synovial fluid contains degenerative neutrophils. L-form bacteria usually cannot be grown from joint fluid via routine culture techniques. Synovial biopsy for culture plus histopathologic evaluation for L-form bacteria is more sensitive than only culture of fluid. Borrelia burgdorferi is almost never isolated by routine culture from joints of dogs with Lyme disease. Use of PCR assays on synovial fluid to amplify DNA of Mycoplasma spp., B. burgdorferi, A. phagocytophilum, and Ehrlichia spp. may prove to be an effective way of documenting the presence of the organisms in the joints of affected animals, but objective data are lacking.
Respiratory System
Lower airway specimens are best obtained by transtracheal aspiration or bronchoalveolar lavage during bronchoscopy. Fine-needle pulmonary aspiration biopsy can be used but carries more risk (see Chapter 11). Bacteria can be isolated from the trachea in some clinically healthy dogs. These bacteria are probably transient; common isolates are listed in Box 15-2. Because many organisms isolated from normal dogs have also been associated with lower respiratory tract inflammation, all transtracheal aspiration samples should be evaluated by culture, antimicrobial susceptibility, and cytology. With cytology, the clinician should look for squamous cells coated with bacteria (which indicate oropharyngeal contamination) (see Figure 11-12). Bacteria should not be considered significant unless accompanied by neutrophilic inflammation. Mycoplasma spp. have been isolated in pure culture from lower airways of patients with clinical signs of respiratory disease.18,71 Culture for Mycoplasma spp. should be performed on all transtracheal aspiration samples; these samples need to be transported to the laboratory in Amies medium or modified Stuart bacterial transport medium. Mycoplasma spp. culture should be specifically requested. Amplification of Mycoplasma spp. DNA from airway secretions by PCR assay can also be used to document infection.
Nasal specimens are best obtained from nasal lavage or core biopsy, or by passing a swab through a sterile otoscope cone (see Chapter 11). The clinician can best obtain pharyngeal specimens using a guarded swab taken during pharyngoscopy. Nasal and pharyngeal cultures can be difficult to interpret because of extensive normal flora in the nasal cavity and nasopharynx (see Box 15-2).
Specimen Transport
For aerobic culture, no special transport medium is required if the swab remains moist and can be inoculated onto the culture medium within 3 hours. Swabs containing liquid or gel transport media are frequently used, however. Routine cultures can be safely stored in transport media at room temperature for up to 4 hours. After this time, overgrowth is a potential problem because of various growth rates of different organisms. Refrigerated, routine specimens can be stored in transport media for at least 2 days. Tissue samples can be refrigerated for up to 2 days. Fluids (e.g., urine) can be safely stored at room temperature for 1 to 2 hours, refrigerated for 24 hours, and refrigerated in transport media for 72 hours.44 Quantitative culture is not accurate for fluids stored in transport media because of artifactual dilution.
Interpretation
Recognizing normal flora (see Box 15-1) is necessary for correct interpretation. Preliminary identification is expected in 18 to 24 hours, and antibiotic sensitivity is reported in 36 to 48 hours. Most aerobic and facultative organisms are identified within 5 days; identification of anaerobic organisms or Mycoplasma spp. may require an additional 2 to 3 days.
Bacterial pathogens commonly isolated from various body systems are listed in Box 15-2. The overlap between resident and pathogenic organisms should be noted.
Bacterial growth from urine obtained by cystocentesis is significant because the bladder is normally sterile. Urine cultures, however, are best interpreted in conjunction with a urinalysis. If growth occurs despite absence of significant pyuria (see Chapter 7), sample contamination, improper sample transport, or diseases causing immune suppression (e.g., hyperadrenocorticism, diabetes mellitus, FIV infection) must be considered. In quantitative culture of urine obtained by catheterization or midstream voiding, greater than or equal to 100,000 colonies/ml is significant. Lower concentrations may be significant in chronic infections or in females. In samples of prostatic fluid obtained by ejaculation, infection is diagnosed if the specimen contains greater than or equal to 100 times more bacteria than the urethral sample.34 Culture of prostatic aspirates may be more accurate.
Diagnostic Tests for Select Bacterial Infections
Bartonellosis, Feline (Bartonella henselae, B. clarridgeaie, B. koehlerae)
Occasional Indications
To date, most cats with clinical bartonellosis have been infected with Bartonella henselae. However, B. clarridgeaie, B. koehlerae, and B. quintana have been identified in some clinically ill cats.13,14 Bartonella henselae, B. clarridgeaie, and B. koehlerae are transmitted among cats by Ctenocephalides felis and so cats with a history of flea infestation are more likely to be infected.20 Most cats exposed to Bartonella spp. maintain subclinical infections; however, fever, uveitis, lymphadenopathy, endocarditis, myocarditis, hematuria, and hyperglobulinemia have been documented convincingly in experimentally infected or naturally exposed cats. Results have been equivocal for a link between Bartonella spp. infection and gingivitis or stomatitis.23 Bartonella testing may be indicated in cats with one or more of these clinical syndromes for which another explanation is not readily apparent.
Analysis, Artifacts, and Interpretation
Circulating antibodies are detected by immunofluorescent antibody assay (IFA), enzyme-linked immunosorbent assay (ELISA), and Western blot immunoassay.13,49 Results of these assays prove previous exposure to a Bartonella spp., but as serologic cross-reaction occurs between B. henselae, B. clarridgeaie, and B. koehlerae, assays based on B. henselae antigens cannot differentiate between the agents. Current Bartonella spp. infection is proven by culture or PCR assay results. Results of some PCR assays or genetic sequencing can be used to identify the species of Bartonella involved with the infection.43 Cats can be infected with more than one Bartonella spp. concurrently. Detection of local antibody production by the eye and documentation of Bartonella spp. DNA in aqueous humor has been used to document uveitis as a result of bartonellosis.51 (See Appendix I for laboratories for infectious diseases.)
Up to 93% of healthy cats exposed to C. felis can be seropositive, and so antibody tests results alone cannot be used to prove clinical bartonellosis.67 Between 3% and 15% of seronegative cats are bacteremic, and so antibody test results cannot be used to exclude Bartonella spp. infection from the differential list. Positive blood culture or PCR results prove current infection but do not document clinical illness. Repeated bacteremia has been detected in experimentally inoculated and naturally infected cats; therefore a single negative blood culture or PCR result does not exclude infection.47 Because of these findings, it is currently recommended to combine serologic test results with those of blood culture or PCR assay results when evaluating clinically ill cats for bartonellosis. (See Appendix I for laboratories for infectious diseases.) If test results are positive and there is no better explanation for the cause of illness, treatment may be indicated. To date, there has been no proven clinical benefit to following Bartonella spp. assay results after positive response to therapy.
Because serologic test results do not accurately correlate with presence of bacteremia and individual culture or PCR assay results can be falsely negative, there is no indication for testing healthy cats for Bartonella spp. infection.14,46 The Centers for Disease Control and Prevention recommends maintaining flea control and avoiding bites and scratches to avoid bartonellosis.45 Healthy cats used for blood donors should be seronegative and culture or PCR negative and should be maintained on flea control products.81
Bartonellosis, Canine (Bartonella vinsonii and Bartonella henselae)
Occasional Indications
Dogs from endemic areas or with an appropriate travel history with unexplained myocarditis, endocarditis, granulomatous lymphadenitis, cutaneous vascular disease, hemolytic anemia, polyarthritis, idiopathic effusions, granulomatous meningoencephalitis, granulomatous rhinitis, or thrombocytopenia should be considered for Bartonella vinsonii testing.12,13 The full spectrum of B. henselae–associated illnesses in dogs has not been determined but appears to be similar to that for B. vinsonii.13,30 Based on seroprevalence studies, rural dogs with fleas or ticks are most likely to be exposed to Bartonella spp.
Analysis, Artifacts, and Interpretation
Circulating antibodies against B. vinsonii and B. henselae are most commonly detected by IFA. Infection can be documented by culture or PCR assay result. (See Appendix I for laboratories for infectious diseases.)
Serologic cross-reactivity between B. vinsonii and B. henselae is variable; therefore individual assays for both agents are indicated. Antibodies can be detected in dogs with and without clinical signs. Seronegative test results make clinical illness caused by B. vinsonii or B. henselae less likely. However, many infected dogs have been falsely negative for Bartonella spp. antibodies. Thus the current recommendation is to combine serologic test results with PCR assay or culture. Bartonella spp. are more difficult to amplify or culture from dog blood than cat blood. The gold standard test for documentation of Bartonella spp. infection is the combination of culture on BAPGM media with PCR assay (Galaxy Diagnostic, Inc).25,59 (See Appendix I: Select Laboratories for Infectious Disease.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree