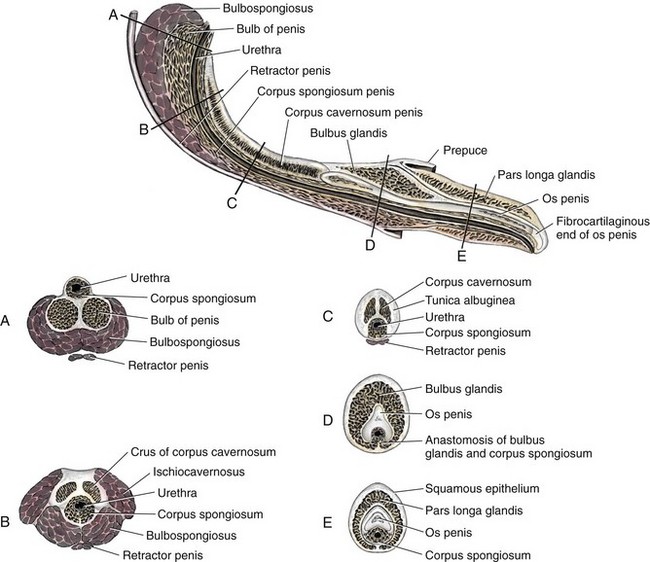
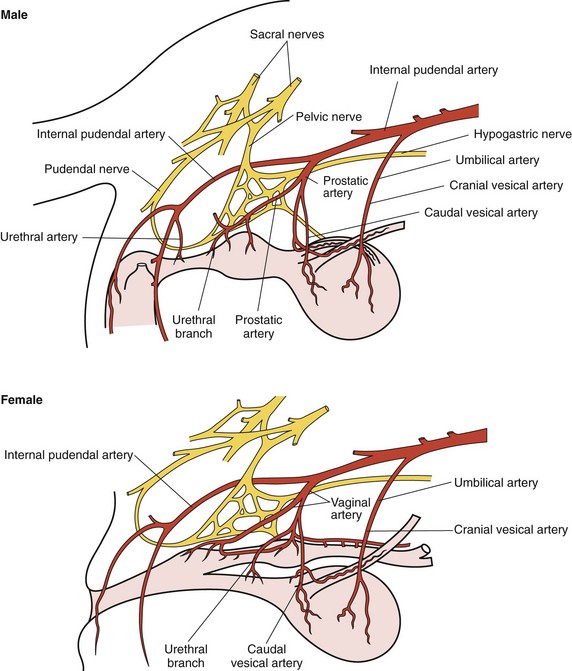
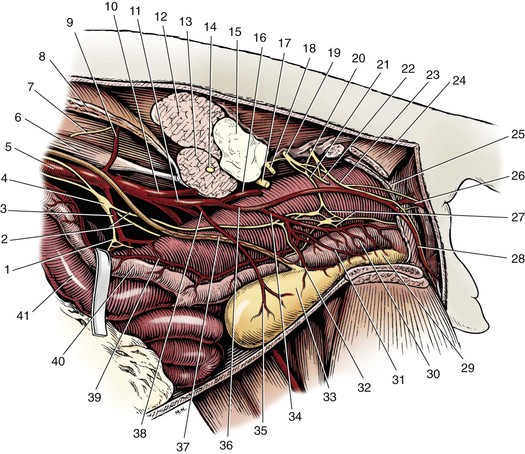
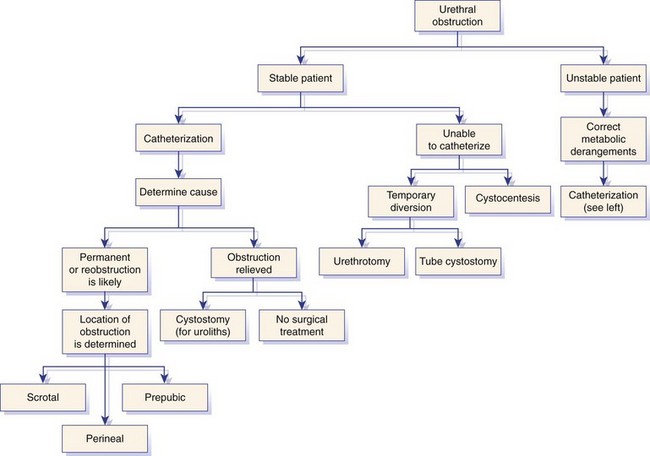
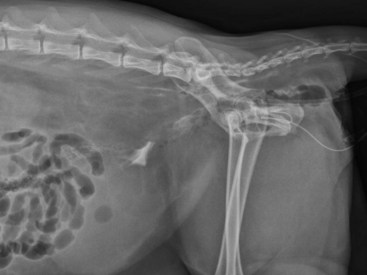
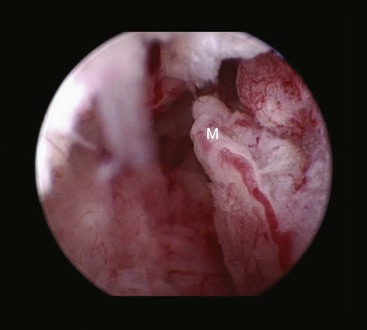
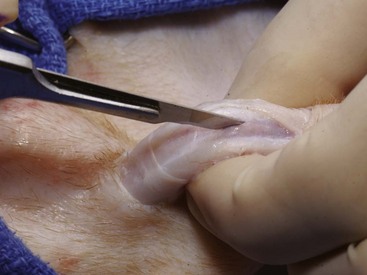
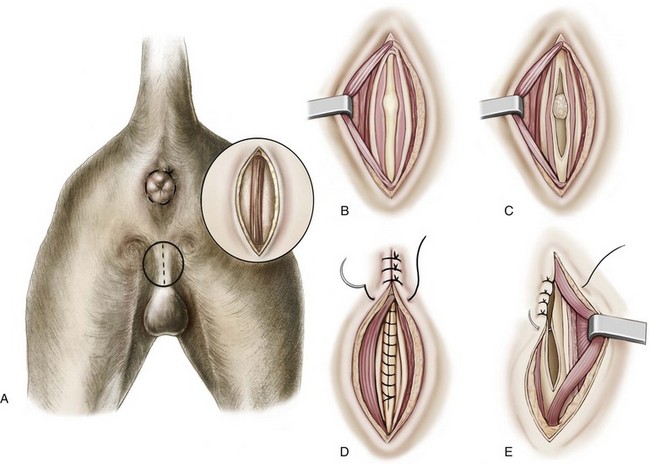
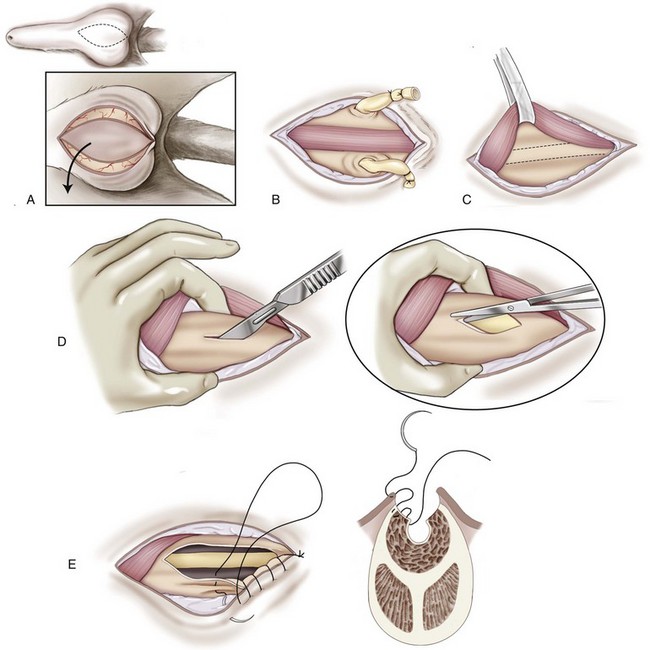
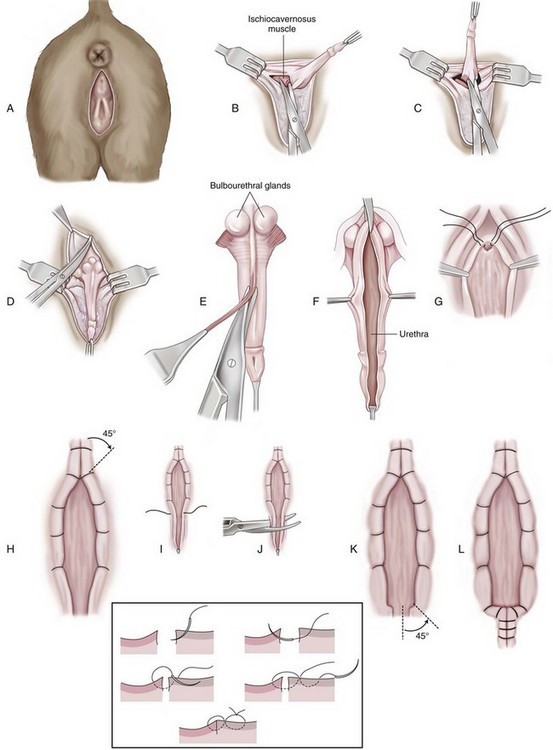
Chapter 117 The urethra is relatively long in male dogs (10 to 35 cm) and varies widely in length and width proximal to the os penis to permit distention with voiding and ejaculation.39 It is divided into three segments: the preprostatic and prostatic sections that lie within the pelvic canal and the cavernous or membranous urethra (pars spongiosa).39 The preprostatic segment extends from the neck of the bladder to the prostate, and the prostatic segment (pars prostatica) passes through the prostate gland. The cavernous portion of the urethra begins at the ischial arch, where the pars spongiosa is joined by the cavernous spaces of the bulbus penis that continue to the urethral termination (Figure 117-1).39 In general, the urethra consists of a mucosal tube surrounded by a vascular submucosa and muscular tunic. Between 20% and 44% of urethral wall volume is connective tissue, and the urethral wall volume increases distally.34 The urethral mucosa is composed of transitional epithelium that forms longitudinal folds when relaxed. The lining becomes stratified squamous epithelium near the external urethral orifice.39 The submucosa consists of a series of vascular sinus channels; within the cavernous segment, the urethral submucosa is a continuation of the vascular erectile tissue of the penile corpus spongiosum.39 Surrounding the submucosa is a thin inner layer of smooth muscle that runs the entire urethral length. Smooth muscle bundles are primarily longitudinally oriented and are continuous with the capsule of the prostate. These taper distally, contributing 0.3% to 12% of the total urethra volume along its length.34,116,117 Smooth muscle of the distal two thirds of the urethra is surrounded by thicker, circularly orientated striated (urethralis) muscle layer.34 This muscle layer comprises 70% of the volume of the wall of the membranous urethra, and unlike in humans, does not diminish with age.117 Functional striated muscle of the dog is reported to contain 3% to 19% of type I (slow-twitch) fibers, depending on breed and regional variation along the length of the urethra; the remaining fibers are type II (fast-twitch).117 Whereas the pudendal (somatic) nerve provides exclusive innervation to striated muscle of the membranous urethra, smooth muscle is innervated by the pelvic (parasympathetic) and hypogastric (sympathetic) nerves (Figure 117-2).32 Urethral vasculature arises from branches of the internal pudendal vessels, including the prostatic, urethral, and penile arteries and veins.39 In contrast to dogs, male cats have a distinct preprostatic urethra that contains three layers of smooth muscle fibers (circular and longitudinal) that contribute to a relatively long internal urethral sphincter.36,119 Although the striated (urethralis) muscle in male cats is relatively thicker in cross-section than that in male dogs, it comprises a shorter functional length of the postprostatic urethra.36 The urethra in male cats also has comparatively more elastic fibers and less stratum cavernosum than in male dogs, and this may contribute to passive urethral resistence.36,119 The age at castration does not affect mature urethral diameter in male cats, although this effect has been reported in some species (cattle, goats, buffalo).101 The urethra in female dogs (see Figures 117-2 and 117-3) is shorter and wider than in males and contains relatively more connective tissue (64% to 70% of total urethral volume).33 Its smooth muscle consists of outer and inner longitudinally oriented layers and a middle circular layer. Circular smooth muscle occupies approximately 25% of the volume of the proximal urethral wall, and this muscle tapers distally and is nearly absent in the terminal urethra.33 Smooth muscle interdigitates with striated (urethralis) muscle fibers in the distal third of the urethra. Striated muscle that remains separate from the pelvic floor completely surrounds the vagina and urethra at the urethral orifice.111 Total musculature is significantly increased to 33% (22% striated; 11% smooth muscle) of urethral wall volume in this region.33 Histologic comparison of urethral composition in spayed and intact female dogs suggests that ovariohysterectomy decreases smooth muscle mass and connective tissue; however, differences were only statistically significant between mean relative volumes in the proximal fourth of the urethra.7 Other investigators found a significantly higher proportion of collagen and, consequently, less muscle tissue in the urethra of gonadectomized female dogs compared with intact dogs.94 Female dogs, regardless of gonadal status, have significantly higher amounts of collagen and less muscle in the urethra than males.94 Collectively, these findings suggest that steroidal hormones may influence morphology of the canine urethra, and this effect may be important in maintaining structural and functional integrity of the lower urinary tract relative to postneutering incontinence (see Chapter 119). The relative length of the urethra of female cats is comparable to that of female dogs; however, the lumen is relatively smaller.35 The urethral wall of cats also contains an appreciable amount of longitudinal smooth muscle and less absolute volume of circular sphincteric muscle and elastic fibers relative to that of female dogs.35 This disparity may influence urethral resistance and suggests that continence mechanics differ among species. The initial assessment and treatment of patients with urethral disease depend on the severity of signs and potential differential diagnoses. Symptoms may be limited to stranguria, dysuria, pollakiuria, or hematuria. Subcutaneous urine leakage results in bruising and, eventually, tissue necrosis. Animals with complete urethral obstruction or significant urine reabsorption eventually develop uremia, metabolic acidosis, and hyperkalemia.107 Severe hyperkalemia results in bradycardia with prolonged QRS width and PR intervals, spiked T-waves, and ventricular arrhythmias. Initial management of patients with suspected urethral obstruction should include evaluation of hemodynamic status, correction of metabolic derangements (see Chapter 116), and urethral catheterization (Figure 117-4). An intravenous catheter should be placed and intravenous fluids and analgesics administered. The fluid choice should be based on analysis of electrolyte and acid–base status; however lactated ringers solution has been shown to be more efficient than 0.9% NaCl for correction of metabolic derangements in experimental urethral obstruction.36a If initial attempts to pass a urethral catheter into the bladder are unsuccessful in animals with urethral calculi or plugs, urohydropropulsion should be attempted. The success of urohydropropulsion can be improved by using urethral catheters of various sizes, lubricating agents, and topical and general anesthesia. Diagnostic and treatment plans can be formulated after a urethral catheter is passed into the bladder. If a urethral catheter cannot be passed, bladder decompression can be maintained by intermittent cystocentesis or insertion of a cystostomy tube (placed under local block and sedation) until the patient is sufficiently stable to perform definitive surgery.25,107 The bladder should be drained to the maximum extent possible to reduce the risk of leakage through the puncture site. A sample of urine should be obtained by cystocentesis or urethral catheterization before administration of antibiotics. Diagnostic imaging of the urethra can present unique challenges, depending on patient size, temperament, and anatomic and functional constraints. Survey radiographs should be evaluated for abnormal urethral location, radiopaque urethral calculi, or concurrent pathology that may have resulted in urethral injury. Plain radiography is rarely diagnostic for the presence of urethral trauma;91,106 therefore, an absence of findings on plain radiographs of a patient with clinical or biochemical evidence of urethral trauma warrants further investigation. Positive-contrast retrograde urethrocystography is the imaging modality of choice for evaluation of urethral lesions.92 Negative-(air) contrast radiography is contraindicated with suspected lower urinary tract trauma because instillation of air into the injured bladder can result in fatal air embolus;1,113 additionally, use of negative-contrast agents rarely demonstrates the location of urethral injury. For positive-contrast studies of the urethra, a balloon-tipped catheter is placed in the distal urethra of males or the distal urethra or vagina (retrograde vaginourethrocystography) of females. Aqueous sterile iodinated contrast material is infused, and the urethra is distended. External compression of tissues around the entry point of the catheter may be required to prevent leakage of contrast material. Ideally, continuous imaging using fluoroscopy should be performed during contrast injection. Orthogonal and oblique radiographic projections can be of value to avoid superimposition of the urethra with skeletal structures. Signs of urethral disease include filling defects and contrast medium extravasation (Figure 117-5).92 Ultrasonographic examination is limited to the extrapelvic urethra but can add complementary information about urethral wall thickness and mucosal surface contour.50 Computed tomographic and magnetic resonance imaging of the normal canine urethra have been described in normal animals; however, the utility of these imaging modalities in animals with urethral disorders has yet to be demonstrated.111,120 Recent advances in equipment and training have resulted in an increased use of cystoscopy and urethroscopy for evaluation and treatment of a variety of lower urinary tract diseases.79 Urethroscopy allows direct observation and biopsy of areas of interest and provides opportunities for intervention (Figure 117-6). Advanced techniques include transurethral cystoscopic dilatation of urethral strictures, mass or polyp resection, laser lithotripsy, submucosal collagen injections, and laser ablation of ectopic ureters or polyps.* The urethra possesses impressive healing capacity; under optimal conditions, urethral mucosa can regenerate within 7 days.14 Critical factors that influence urethral healing are mucosal continuity and urine extravasation.13 Urethral tissue becomes markedly edematous with manipulation and increasing duration of surgery, obscuring identification of tissue layers that may result in suboptimal suture placement and poor tissue apposition.13 Use of magnification loupes may improve identification of the urethral mucosa. Achieving uncomplicated healing with minimal fibrosis is essential to minimize the occurrence and severity of urethral strictures. Narrowing of the urethral diameter must reportedly exceed 60% before an animal shows clinical signs;69 nonetheless, urethral stricture formation is a recognized potential sequela to urethral trauma and surgery. Conservative therapy is indicated for minor urethral injuries, such as contusions, small lacerations, and perforations that result from catheterization.23 Large urethral defects will heal spontaneously by second intention provided that a strip of urethral mucosa remains intact across the damaged tissue and the flow of urine is diverted.121 It is often possible to pass a urethral catheter in a retrograde direction in the presence of a urethral laceration; postcatheterization positive-contrast radiography is recommended to confirm that the catheter has not been passed through a defect in the urethra. Complete transection of the urethra requires surgical intervention. If the severed urethral ends have retracted, it may be necessary to perform a cystotomy to pass a catheter in an antegrade direction to identify the distal end of the proximal urethral segment. Traumatized edges of the urethra are debrided before primary repair of the defect. Dissection must be sufficient to prevent tension across the anastomosis because tension across the suture line promotes stricture formation.62 Similarly, accurate approximation of urethral mucosa is crucial to promote primary wound healing and minimize fibrosis and scar formation. The decision as to whether or not to leave a urethral catheter in place after surgery is, to some degree, a matter of surgeon preference. Repeated exposure of submucosal tissue to urine may promote formation of scar tissue, reducing the elastic qualities of the affected region of urethra.90 Although preventing contact of the wound with urine may decrease inflammation, the presence of the catheter can promote inflammation.69 Some reduction in urethral luminal diameter at the site of surgical repair is anticipated, regardless of whether or not a catheter is left in place after surgery. The ideal suture material should maintain tensile strength and tissue apposition until wound repair is satisfactory and should then undergo rapid total absorption at a dependable rate. In general, the lower urinary tract is expected to heal rapidly. In experimental dog models, suture in healthy bladder tissue was covered by epithelium within 5 days.51 Although urethral healing was not described in that study, it is assumed to heal in a similar manner. Inflamed, infected, or damaged urothelium may not reepithelialize as quickly, and exposure of suture materials to urine, especially in the presence of infection or altered pH, may accelerate the loss of tensile strength through increased hydrolysis.105 A variety of suture materials are available and are effective for urethral surgery. In general, most synthetic absorbable sutures retain adequate strength for urethral repair. Monofilament synthetic absorbable suture material, typically either 4-0 or 5-0 USP sizes, with a swaged-on needle, is appropriate in small animals.59 Polydioxanone (PDSII; Ethicon), polyglyconate (Maxon; Syneture), poliglecaprone 25 (Monocryl; Ethicon), and glycomer 631 (Biosyn; Syneture) are all acceptable suture materials. Experimentally, however, the tensile strength of poliglecaprone 25 is lost at a relatively rapid rate in the presence of urine.45 This suture may be inappropriate if delayed healing is anticipated, although clinical trials are lacking. Use of braided multifilament suture materials such as polyglactin 910 (Vicryl; Ethicon) has been questioned because it may harbor bacteria and undergo rapid degradation in urine;105 however, anecdotal evidence supports its successful use in urethral tissue. As mentioned previously, postoperative urethral catheterization is somewhat controversial. Although exposure of traumatized urethral tissue to urine may result in delayed healing and increased periurethral fibrosis, urethral catheters initiate a certain amount of inflammation and can promote ascending infection.70,71,83 Diversion of urine can be provided by transurethral catheterization or placement of a cystostomy catheter. At least one study suggests that based on radiographic, dynamic, and histologic evaluation, the type of urinary diversion used does not influence urethral function or healing.30 Urinary diversion into the gastrointestinal tract has been described in dogs but is associated with a prohibitively high complication rate.110 If performed, urine diversion should be maintained until epithelization is complete. Epithelialization for small defects that retain some mucosal continuity may occur as soon as 7 days after trauma. Some authors recommend maintenance of an intraurethral catheter for at least 3 weeks after surgery or injury of the urethra;97,121 however, it is unclear whether prolonged (weeks) maintenance of an intraurethral or cystostomy catheter decreases stricture formation.69,80 If delayed healing is a concern, urethral continuity can be evaluated with positive-contrast urethrography to verify mucosal integrity. Urethrotomy is the creation of a temporary opening in the urethra. It is most commonly indicated for removal of calculi that cannot be shifted by hydropropulsion techniques or to temporarily bypass other types of obstruction. Urethrotomy may also be performed to expose obstructive lesions or masses for biopsy. The urethrotomy site is based on the location of the obstructive lesion. The prescrotal region is preferred in male dogs because of the urethra’s superficial position and limited surrounding cavernous tissue (see Figure 117-1); additionally, it provides the option of revision in a more proximal location if complications arise. Perineal or prepubic urethrotomy can also be performed in male dogs and cats. The procedure is similar to prescrotal urethrotomy (described below), with the exception that the urethral incision in the perineal or prepubic locations must invariably be closed with sutures. When calculi can be dislodged and flushed back into the bladder, performance of cystotomy is always preferable to urethrotomy to minimize the potential for complications. The patient is placed in dorsal recumbency with the pelvic limbs extended and abducted. The abdomen is clipped and aseptically prepared to allow performance of cystotomy if it becomes necessary. The scrotum should be included in the operative field if conversion to a scrotal urethrostomy is considered a possibility. The prepuce should be irrigated with dilute antiseptic solution and draped into the surgical field to allow catheterization. Retrograde urethral catheterization is performed to hydropulse calculi that could not be shifted before surgery, facilitate identification of the urethra, and determine the position of the obstruction. A 1- to 2-cm incision is made on the ventral midline caudal to the os penis and cranial to the scrotum (Figure 117-7). Dissection is continued through the subcutaneous tissue to expose the penile shaft. Paired retractor penile muscles overlying the urethra are retracted laterally. The urethra is identified as a purple structure, 3 to 4 mm wide, bordered on either side by white corpora cavernosa. A longitudinal incision is made through the midline of the urethra over either the catheter or calculus. Profuse hemorrhage often occurs upon urethral incision because of the vascularity of corpus spongiosum encircling the urethra and can typically be controlled with direct pressure. Calculi are removed, and a urethral catheter is inserted into the urethral orifice and advanced beyond the urethrotomy site into the bladder. A cystotomy can be performed to remove large calculi that remain within the bladder. The urethra is catheterized in antegrade and retrograde directions and flushed with sterile fluid to ensure that all calculi have been removed. The decision as to whether or not to suture the urethrotomy site is based on surgeon preference. Although primary and second intention healing of urethrotomy sites are similar, more hemorrhage has been observed when the urethrotomy is not sutured.118,122 Suturing is performed with 4-0 or 5-0 monofilament absorbable material in an interrupted or continuous pattern. Generous application of petroleum-based jelly around the urethrotomy (but not in the wound itself) may reduce urine scalding and scrotal dermatitis that can occur when urethrotomy sites are left open. In male dogs, urethrostomy can be performed at prescrotal, scrotal, perineal, and prepubic locations. Scrotal urethrostomy is preferred because the urethra in that region is superficial and relatively wide, and less hemorrhage results at that site than at other locations. Prescrotal and perineal urethrostomies in male dogs may result in urine scalding along the scrotum or medial surface of the hindlimbs. The procedure for prescrotal or perineal urethrostomy (Figure 117-8) is essentially similar to scrotal urethrostomy (described below), although the retractor penile muscle is not present in the perineal location. Urethrostomy can be performed in male cats in either a perineal or prepubic location. Urethrostomy in female dogs or cats is limited to a prepubic location. An elliptical incision is made around the base of the scrotum in intact male dogs or the scrotal remnant in castrated male dogs (Figure 117-9). Care is taken to leave adequate skin on the lateral aspects of the incision to allow a tension-free closure. Castration is performed in a routine manner through the skin incision, and the testes and scrotum are removed. The retractor penile muscle is freed from its attachment to the underlying urethra and retracted laterally. Several interrupted sutures of 3-0 or 4-0 absorbable suture material are placed from the tunic of the penis to the subcutaneous tissue on either side of the intended urethrostomy site to maintain the penis and urethra in a superficial position. A small incision is made through the ventral midline urethra with a scalpel blade. It is crucial that the incision into the urethra be made on the midline to limit hemorrhage and to provide sufficient urethral tissue on either side of the urethrostomy site to suture to the skin. Care must be taken to avoid lacerating the dorsal urethral surface; therefore, incision over a catheter is preferable. The incision is extended 2.5 to 4 cm (~five to eight times the urethral diameter) with fine scissors to ensure adequate size of the stoma. The incision may initially appear excessive; however, the stoma will reduce to one half to two thirds its initial length after healing is complete.107 The urethra often bleeds profusely from the incision, but bleeding decreases as sutures are placed. The urethra is sutured to the skin using 4-0 or 5-0 monofilament suture material in a single-layer simple interrupted or simple continuous pattern. The use of absorbable monofilament sutures (e.g., poliglecaprone 25 or glycomer 631) has been described,107 but the authors have a strong preference for the use of monofilament nonabsorbable sutures because of the inconsistent rate of absorption and loss of absorbable suture placed in this location. The suture is passed through the mucosa and fibrous tissue of the urethral wall but includes only dermis and epidermis of the skin. This facilitates precise approximation of the skin to the wall of the urethra. On completion of the scrotal urethrostomy, it should be possible to advance a catheter from the urethrostomy site into the perineal urethra and bladder. An Elizabethan collar or other restrictive device is placed on the dog before recovery. Nonabsorbable sutures are removed 10 to 14 days after placement, and sedation may be required for suture removal. Absorbable sutures are usually sloughed by the patient within 1 to 2 weeks after Elizabethan collar removal. The most common complication of scrotal urethrostomy is persistent hemorrhage. Reportedly, use of a simple continuous pattern for urethrocutaneous apposition reduces the average duration of active bleeding from 4.2 days to 0.2 days.20,85 In the authors’ experience, there is no advantage associated with use of a continuous pattern for closure of urethrostomy provided sutures are placed appropriately. Bleeding associated with urination is detected for an average of 3 to 5 days after urethrostomy and usually is self-limiting. Hemorrhage that persists for more than 10 to 14 days after surgery may necessitate revision of the urethrostomy site. Intermittent urine scald, recurrent urinary tract infections, and recurrent obstruction from struvite calculi were each noted in 20% of dogs after scrotal urethrostomy in one report.85 Stricture is rare and is likely a result of a previous insult (e.g., calculus), poor surgical technique, or postoperative self-trauma. Perineal urethrostomy for treatment of distal urethral obstruction and injury was first reported in 1963.27 Various techniques and modifications have been described for urethrostomy in male cats,60,99 but the Wilson and Harrison technique is most commonly used22,126 and is described in this text. The penis is freed of its pelvic attachments, the urethra is transected cranial to the penile portion, and the pelvic urethra is sutured to the perineal skin.126 A modification of the perineal urethrostomy entailing anastomosis of the urethra to a remnant of the preputial mucosa has been described in a series of male cats.128 The authors suggested that this procedure improves cosmesis and decreases the incidence of postoperative stricture and bacterial cystitis. More experience with the technique is required to assess these claims, however, and this modification has not been widely adopted to date. Technique in Ventral Recumbency: The cat is positioned in ventral recumbency (Figure 117-10) with the hindquarters elevated over a padded surface. The cat should be positioned to minimize diaphragmatic compression by the abdominal viscera. A rolled towel can be inserted between the cat’s cranial thorax and the tilted table to reduce abdominal pressure. A purse-string suture is placed in the anus, and a urethral catheter can be placed.
Urethra
Anatomy
Male Dogs
Male Cats
Female Dogs
Female Cats
Patient Evaluation
Diagnostic Imaging of the Urethra
Surgical Principles
Partial Defects
Complete Transection
Catheter Placement After Repair
Suture Material
Urinary Diversion
Surgical Procedures
Prescrotal Urethrotomy
Urethrostomy
Scrotal Urethrostomy in Male Dogs
Perineal Urethrostomy in Male Cats
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Urethra
Only gold members can continue reading. Log In or Register to continue